The global population is expected to reach nearly 10 billion by 2050, and as it increases, the need to produce enough food for all people also grows. Traditional protein sources, such as livestock and soy, put extra pressure on the environment, as they require vast amounts of land, water, and energy while contributing to Greenhouse gas (GHG) emissions.
The accumulation of these GHGs accelerates climate change, putting extra stress on agricultural systems and threatening food security. As a result, we are faced with a dual challenge: on the one hand, we need to produce more protein to meet the rising demand, and on the other hand, we need to reduce the environmental footprint of our food systems.
Microorganisms as an answer
Microbes are remarkably efficient at utilizing carbon and energy and transforming it into biomass. Microbial metabolism is highly diverse, and depending on the microorganism, substances such as sugars, or gases, such as methane, hydrogen, or carbon dioxide and monoxide (gas fermentation) can be utilized by the cells. This ability means that they can even transform GHG into organic matter, effectively using emissions as feedstock.
Compared to conventional protein sources, microbial production requires far less land and water, making it

more environmentally friendly. Bioreactors are designed to maintain controlled conditions, therefore reducing the impact of climate or geography, increasing the scalability and reliability of the process. On top of that, it also offers a pathway to mitigate GHG emissions, instead of adding to them. As a result, we can produce food products while simultaneously helping to address global warming.
What is Single-Cell Protein?
The term “single cell protein” (SCP), also known as “microbial protein” (MP), refers to edible microbial cells. It can be either pure or mixed cultures from bacteria, yeast, fungi, or algae, and the biomass or protein extract can be used as an ingredient or substitute for protein-rich foods. Microorganisms present the advantage of growing rapidly, being able to reach high cell densities under the right conditions. Once cultivated, the biomass can be harvested and processed into protein-rich powders suitable for food or feed applications. SCP, in order to be viable for consumption, not only needs to have high nutritional value, but also needs to have a low concentration of nucleic acids (~8-12%), which can result in health issues such as kidney stones and gout. Furthermore, economic viability is fundamental to determining whether the product will be successful, including feedstock viability, scalability, and processing.
SCP typically contains between 50-80% protein by dry weight and is rich in essential amino acids. SCP can be used in multiple ways:
- Animal feed: especially valuable for aquaculture and livestock, where conventional feed sources are resource-intensive.
- Human food: from dietary supplements to meat alternatives
- Other applications include producing tailored amino acid profiles for specific pharmaceuticals or nutritional applications
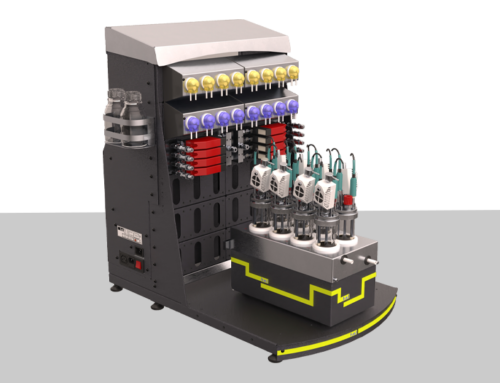
Production occurs in bioreactors, where physico-chemical parameters, such as pH, temperature, dissolved oxygen, or gas flow, are strictly monitored and controlled. For researchers, benchtop systems, such as H.E.L Group’s BioXplorer, offer a flexible platform to optimize these parameters, proving ideal conditions for testing new microbial strains and exploring feedstocks. Precise control and real-time monitoring facilitate reproducible results and consistency between batches.
Banks, M., Johnson, R., Giver, L., Bryant, G. & Guo, M., 2022. Industrial production of microbial protein products. Current Opinion in Biotechnology, 75, p.102707. https://doi.org/10.1016/j.copbio.2022.102707
Current Applications: Microorganisms and Products in Use
In the last few years, the production of SCP has boomed, and several microorganisms and companies are taking over the market. Different microbes are being used to produce SCP, each with unique advantages:
- Bacteria
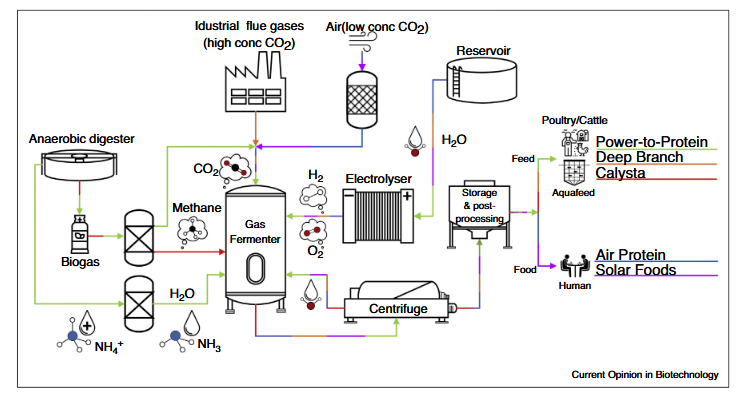
- Cupriavidus necator can utilize hydrogen and CO₂, making it a strong candidate for gas fermentation processes.
- Methylococcus capsulatus, a methane-oxidizing bacterium, is already being used to produce animal feed. Companies such as Calysta and Unibio are pioneering methane-to-protein platforms
- Yeasts
- Saccharomyces cerevisiae (the same yeast used in brewing) and Candida utilis have long histories in fermentation and food production. They remain relevant SCP candidates thanks to their robustness and established safety profiles.
- Algae
- Species like Chlorella and Spirulina are commercially available as dietary supplements. They are protein-rich and contain valuable pigments and fatty acids, though their large-scale cultivation still faces cost challenges.
- Fungi
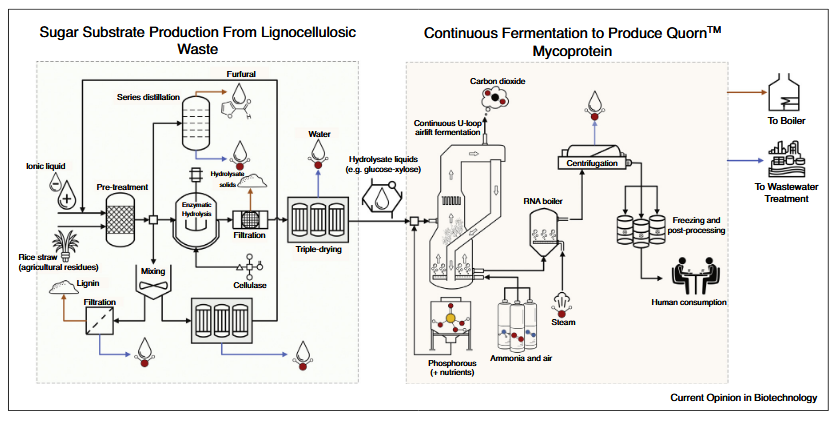
- Fusarium venenatum is the microorganism behind Quorn™, a well-known meat substitute. This success story demonstrates the potential of fungal SCP to reach mainstream consumers.
Several companies have been pushing the boundaries of microbial protein:
- Solar Foods produces Solein®, a protein derived from CO₂ and hydrogen, marketed as “protein from air.”
- LanzaTech uses gas fermentation technology to convert industrial waste gases into fuels and chemicals, while also exploring protein applications.
- Calysta focuses on methane-based SCP, producing protein-rich feed ingredients for aquaculture.
These examples prove how microbial fermentation is becoming more commonplace in the current market, with growing momentum across both feed and food sectors.
Banks, M., Johnson, R., Giver, L., Bryant, G. & Guo, M., 2022. Industrial production of microbial protein products. Current Opinion in Biotechnology, 75, p.102707. https://doi.org/10.1016/j.copbio.2022.102707
The Future of Gas Fermentation and SCP
Single-cell protein is becoming more prominent, and its future looks promising. Advances in gas fermentation, using GHG as feedstock, paired with the use of renewable energy, point towards a sus system where CO2 and green hydrogen can be transformed into protein. This represents a shift in the current paradigm: food production becoming decoupled from farmland, independent of climate, and sustainable.
However, the path ahead is not free of challenges. Scaling up gas fermentation requires addressing technical issues such as gas-liquid mass transfer and ensuring consistent product quality. Regulatory approval is also crucial, and it is particularly stringent, and for good reason, for human food applications. Nevertheless, the trajectory is clear: microbial protein is set to play a growing role within the global food system.
For researchers and developers, tools that facilitate precise experimentation and scale-up processes are critical. Bioreactor platforms provide the perfect environment for this, where gas fermentations can be tested, optimized, and scaled up to achieve industrial-scale applications.
Conclusion
Feeding a growing human population is a multifaceted challenge; the limitation of space and resources, the variability of climatic conditions, and a warming planet make a complex problem. Microbial fermentation offers an intriguing solution, optimizing limited resources while reducing the impact of climate change by removing carbon from the atmosphere and producing protein. With single-cell protein, we can produce nutritious, sustainable food and feed with a fraction of the resources required by traditional methods.
From bacteria that thrive on methane to fungi that are already strong substitutes for meat, microorganisms keep proving their value as sustainable protein factories. Companies across the globe are demonstrating that this approach can be scaled and commercialized, while researchers continue to explore new strains, feedstocks, and bioreactor designs.
Ahead of us, there is still innovation to be achieved, underpinned by investment, and propped up by collaboration. But the potential is extraordinary: a future where feeding people and protecting the planet go hand in hand. And at the centre of this transformation are the microbes and the technologies that help us harness them.