Safety is paramount in the never-ending quest to achieve better batteries with higher capacities, longer lifespans, and faster charging times. The news is dotted with innumerable incidents in which battery failure has resulted in fires, reaching catastrophic results in some cases. Decomposition reactions in the electrodes and electrolytes produce heat, accelerating these reactions and resulting in thermal runaways.
Testing is fundamental to avoid such dangerous scenarios. Delimiting safe operational limits and the consequences of a battery failure is critical. However, no single technique can provide all the information required to establish these ideal working conditions.
This blog post will discuss and compare different evaluation techniques, focusing on isothermal calorimetry, environmental chambers, and adiabatic calorimetry.
What is isothermal calorimetry?
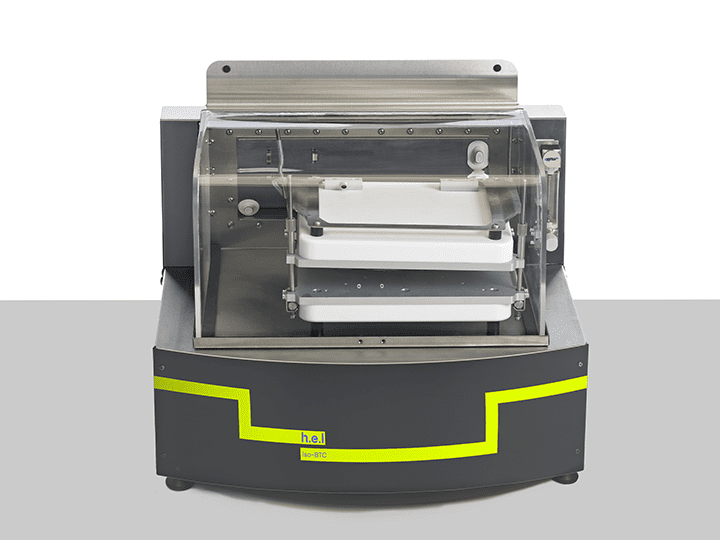
iso-BTC – an example of an isothermal calorimeter
Calorimetry is the science that studies the heat released or absorbed by a process. Isothermal calorimetry analyses the behavior of the process of interest at defined constant temperatures. This is particularly relevant in the study of energy-storing devices. As a battery charges and discharges, energy can be exchanged with the environment, and its value and behavior will change depending on the temperature at which the battery is. This has severe ramifications. On the one hand, operation under sub-optimal conditions may result in slower charging and faster discharge times and can impact the battery’s lifespan.
Isothermal calorimeters with charge-discharge units monitor this process and measure the battery’s thermal behavior. This helps identify the ideal operational conditions and the consequences of operating the battery outside those conditions.
What are environmental chambers?
Environmental chambers operate following a similar principle to isothermal calorimeters. The battery is exposed and maintained at defined temperatures to characterize its behavior. However, actual conditions might differ from testing conditions. As a result, environmental chambers and their capabilities to test other variables are very useful tools to verify longevity.
Apart from temperature, environmental chambers are also suited to test other environmental factors, such as relative humidity (RH). The combination of these factors leads to accurate simulation of real-life situations. As a result, the consequences of the failure can be assessed, and mitigation strategies can be put in place. Environmental testing allows for categorizing batteries in one of 8 levels, according to EUCAR (European Council for Automotive R&D). For a battery to be considered safe for vehicles, it must fall under the 4th category or less. This means that there may be a loss of electrolyte mass. However, no rupture or explosion of the battery may occur in these batteries.
What is adiabatic calorimetry?
Adiabatic calorimetry, like isothermal calorimetry, measures heat exchange. However, the difference between these two methodologies is that while the temperature is maintained constant in isothermal calorimetry, there are no heat losses toward the environment in adiabatic calorimetry. This allows for an in-depth characterization of the thermal events.
Whereas isothermal calorimetry and environmental chambers allow for the characterization of the normal functioning of the battery, determining the limits where the battery is safe to operate, adiabatic calorimetry goes a step further.
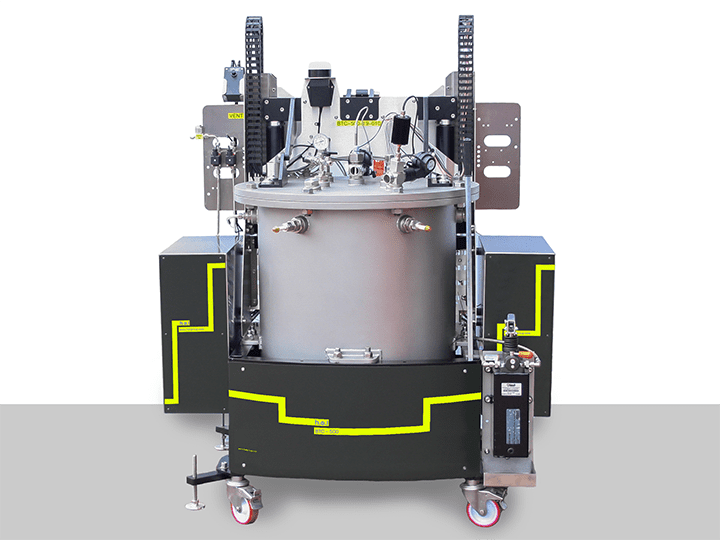
BTC-500– an example of an adiabatic calorimeter
Adiabatic calorimetry enables an understanding of the behavior of the battery when it goes into thermal runaway and what the worst-case scenario is. Fundamental information that can be obtained by adiabatic calorimetry includes:
- The temperature at which the thermal runaways start (exotherm)
- The heat generated by the thermal event
- Temperature rise
Understanding the risks of battery failure and the point at which the dangerous zone starts enables scientists and engineers to develop strategies to avoid reaching that point and, secondly, to diminish the consequence of such an event. These might include cooling elements to prevent reaching the exotherm and insulation to mitigate the damaging effects of the thermal runaway.
A tool for every step
Different techniques provide different insights into the behavior of batteries. Isothermal battery testing is an incredibly powerful technique that allows an understanding of how the normal function of a battery is affected by different temperatures. Different processes, such as charging and discharge, efficiency, and longevity, can be affected by the temperature at which the device is functioning. However, temperature does not only affect the efficiency and functioning of batteries. Other environmental factors, such as relative humidity (HR), alter the capabilities of batteries. Environmental chamber testing provides fundamental information, but their increased resilience permits failure analysis. This analysis is crucial to understand whether the physical integrity of the device is compromised should a thermal runaway happen. Legislation, such as EUCAR, is designed to keep users safe and has very astringent requirements that must be tested in such equipment. Finally, adiabatic calorimetry is greatly suited to determine the behavior of batteries once they enter a thermal runaway. This provides insights into the exotherm’s evolution and the battery failure’s consequences.
In a greener world where we try to avoid fossil fuels, batteries are in continuous demand. Higher capacities, fast charging times, and longer lifespans might come with higher risks. However, isothermal and adiabatic calorimetry and environmental chambers provide the testing capabilities to create safer energy storage devices suitable for current world needs.