It is interesting how inspiration happens sometimes. As I was sipping coffee and deciding what topic to bring to this edition of “The Modern Scientist Blog” it struck me. I could combine two of my passions: coffee and calorimetry. In this blog we look at how materials as simple and humble as a Styrofoam coffee cup and a thermometer can help us understand concepts as abstract as calorimetry.
But what is it that we can measure using such simple equipment? The answer is enthalpy, which might sound like a term that physicists love using but does not have any impact on day-to-day life. However, that is not true, and enthalpy is a constant in our lives. The energy at work warms our homes, allows us to cook food, or fuels our cars. In all these processes, heat transfers, and that is where the word, enthalpy, comes to play.
So, how does enthalpy exactly work? Imagine a cold winter day. The house is getting cold, and we must make it more habitable and warmer. So, we crank up the heating. If it were a gas boiler, a quick exothermic reaction – combustion – would occur. The energy is then released into the surroundings, in this case, our home. In terms of enthalpy, we can say that the change in enthalpy (ΔH) is negative because the energy is being lost by the system (the heater) and gained by the surroundings, the room.
Understanding the concept of enthalpy means, we can learn how energy is transferred and how it can impact our daily lives. So, let us dive into the science behind enthalpy and how we can quantify heat transfer.
But what is enthalpy?
Enthalpy (H) can be described as the total energy of a system, which includes both the internal energy (U) of the system plus its pressure (P) and volume (V), and its represented by the following equation:
H = U + PV

Enthalpy is a fundamental concept, as it allows for the quantification of energy changes that can occur in chemical reactions. Whereas pressure and volume are easily measurable, the total enthalpy of a system cannot be measured directly, as the internal energy generally contains unknown or very difficult-to-determine components. In most everyday situations, the change in enthalpy occurs at constant pressure, so any enthalpy changes are linked to the heat absorbed or produced by the reaction. This type of calorimetry is called isobaric calorimetry. Suppose the cup we use in this experiment is very insulating, like in the case of a Styrofoam cup. In this case, the type of calorimetry we are using is adiabatic calorimetry. This means that the reactor (in our case, the cup) keeps all the heat released or absorbed by the reaction, allowing us to measure it.
So, if there are changes in the enthalpy but no changes in the volume, then it would mean that all the enthalpy changes will be linked to the heat released or produced during the reaction. As the reaction progresses, it will either generate heat, increasing the temperature in its surroundings (exothermic reaction), but it can also be the case that the energy absorbs heat (endothermic). The heat can be easily measured using the following equation:
q = mwater x Cwater x (Tf-T0)
In this equation, m is the mass of water inside of the cup, Cwater is the thermal capacity of water and Tf – T0 is the final temperature minus the initial temperature. In this case, we can assume that all the heat will be transferred into the water, which is why we use the thermal capacity of water, and therefore the water temperature will change.
Having our Styrofoam cup equip ped with a thermometer and knowing the amount of water we have put in our cup, we can calculate the heat exchange and understand how much the enthalpy has changed.
Professional calorimeters

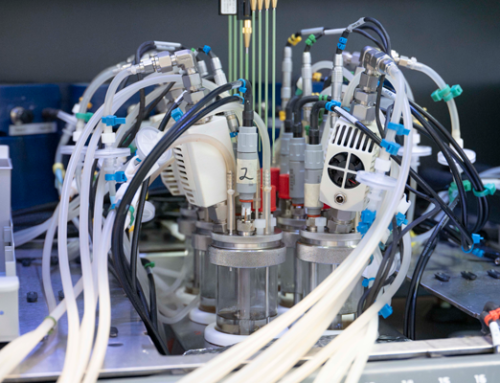
Professional calorimeters, like H.E.L’s Simular, function similarly to the Styrofoam cup. Instead of having Styrofoam as an insulator, the reactor is surrounded by a jacket containing a fluid, such as oil, that will absorb or provide the heat in the reaction. The calorimeter will have several heaters and thermometers that allow for the detailed tracking of the reaction.
There are different ways to measure heat in calorimeters. For example, analyzing heat flow. In this case, the reaction produces or absorbs heat, making the temperature in the fluid in the jacket change. Similar to the Styrofoam cup, the changes in
temperature in the fluid will determine how the enthalpy changes. Other operating methods, such as power compensation, aim to keep the temperature in the reactor constant. For this, the fluid in the jacket will be heated to a specific temperature. As the reaction progresses, for the jacket temperature to remain the same, the heater must stop (if the reaction is exothermic) or increase the energy (if the reaction is endothermic). In this case, instead of measuring the different temperatures, the value that the reactor will measure is how different the power supplied to the heater will be, and it will be the same as the heat change due to the reaction.
Enthalpy, from abstract concept to concrete reality
Enthalpy can seem such an abstract concept, but today we have seen how such an ethereal idea has ramifications in our day-to-day lives – from heating our homes to brewing a cup of tea. We have seen how we can measure the changes in the energy of a system using simple materials, like a coffee cup and a thermometer. But also how the same fundament is used in professional calorimeters.
The calorimeter data have important applications (you can check some on these blog posts) and help us understand the principles at work from common phenomena to help us drive advancements in technology and industry. As we better understand how energy is exchanged, we enhance our capacity to harness and manipulate energy, opening up new opportunities for the future. This helps us create a healthier, safer, and more sustainable world.