Hydrogenation reactions are incredibly common nowadays. From your breakfast (margarine) to the fuel in cars (green diesel) to face creams (hydrogenated oils) we use before going to sleep, products of hydrogenation are commonplace in our lives. This chemical transformation is behind the texture of processed foods, the shelf-life of cosmetics, and the efficiency of alternative fuels.
Hydrogenation has become one of the cornerstones of industrial chemistry. First developed in the early 19th century by Paul Sabatier, it revolutionized food and soap processing and today plays a crucial role in pharmaceuticals, petrochemicals, agrochemicals, and sustainable energy solutions. It is one of those essential yet invisible processes that quietly supports our modern way of life.
What Is Hydrogenation?
Hydrogenation is a chemical process that reduces the double and triple bonds in molecules, often improving textures, stability, and providing unique characteristics. Unsaturated bonds are reduced as the molecule of interest reacts with hydrogen, usually in the presence of a metal catalyst such as palladium (Pd), platinum (Pt), rhodium (Rh), or nickel (Ni).
Some of these catalysts share the same catalytic mechanism: adsorbing hydrogen first, followed by the substrate. Their close proximity on the catalyst surface facilitates the reaction. However, this same surface can accumulate excess hydrogen, which can react with oxygen in a highly exothermic and potentially dangerous reaction.
Hydrogenation is used across a wide range of applications. For example, hydrogenating vegetable oils turns them from a liquid to a semi-solid, improving organoleptic properties in food products and extending shelf life. In the pharmaceutical industry, hydrogenation is used to modify drug molecules to enhance bioavailability or stability. Even in fuel production, the hydrogenation of biomass-derived oils is essential to creating renewable diesel alternatives.
The Inherent Risks of Hydrogen
The primary safety issue in reactions involving hydrogen gas is its pyrophoric nature, which can lead to fire and explosions. One only needs to remember the Hindenburg Disaster to understand the magnitude of the explosion this gas can produce. Other infamous incidents involving hydrogen include the Challenger Space Shuttle Disaster and the Fukushima Daiichi Nuclear Disaster.
Some of the physicochemical characteristics of hydrogen add further layers of risk. Its small molecular size and dissociation capabilities at high temperatures make it highly diffusive and prone to leaks. Hydrogen flames are nearly invisible, emitting little radiation and being difficult to detect by eye. Compounding this, it has a very wide flammability range (4–76%) and extremely low ignition energy. Hydrogen is also colorless, odorless, tasteless, and an asphyxiant, making accidental exposure and ignition easier and more dangerous.
Added Risks in Hydrogenation Reactions
Hydrogenation reactions carry risks beyond the inherent properties of hydrogen gas. Several factors contribute to how safe or unsafe the process is:
- The nature of the catalyst
- The purity of reagents and potential contaminants
- The stability of intermediates
- Physical conditions, such as temperature and pressure
The greatest hazard in hydrogenation processes is the inadvertent creation of flammable mixtures. Exposure to air, oxygen, or a spark can result in detonation and fire. Furthermore, toxic gaseous byproducts, such as carbon monoxide, nitrogen oxides, or sulfur dioxide, can form and pose serious health risks if inhaled.
Even trace contaminants—like moisture, peroxides, or air—can drastically alter the safety of the process. Certain metal catalysts, when dry, can ignite on exposure to air. The potential for thermal runaways is real, especially when scale-up is not carefully controlled.
Making Hydrogenation Safer
At the lab scale, hydrogenation is often carried out using round-bottom flasks with balloon setups, pressure vessels, or shakers. While these traditional methods are widespread, they come with limitations—most notably, they require intensive manual handling and can introduce significant safety risks.
Modern automated equipment offers a safer alternative by minimizing human intervention and providing tighter control over critical parameters such as pressure, temperature, and gas dosing.
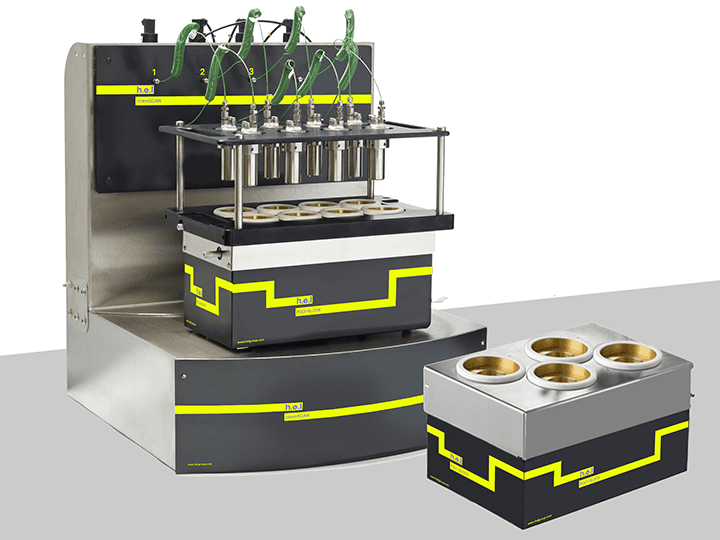
H.E.L.’s ChemSCAN system is a good example. Designed for parallel hydrogenation, it combines automated control with real-time monitoring to reduce user exposure to hazardous gases. This setup enables safer, more consistent optimization of multiple reactions at once.
ChemSCAN
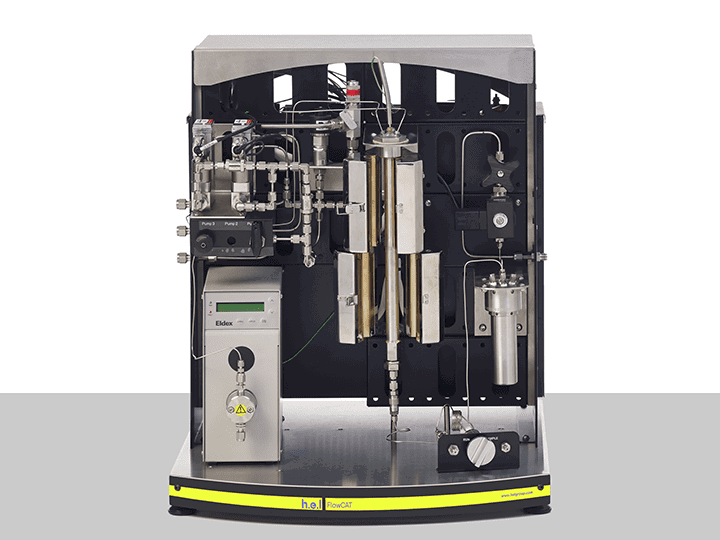
FlowCAT is another automated solution, tailored for continuous hydrogenation. Its tubular reactor design ensures hydrogen flows steadily through the system, which greatly reduces the risk of gas accumulation. Additionally, it delivers excellent heat transfer, a critical feature for safely managing exothermic reactions that demand tight temperature control.
Even with advanced equipment, certain safety practices remain essential. When working with glassware, it is paramount to ensure it is free of cracks and contamination. Use a fume hood to prevent hydrogen accumulation, and avoid open heating systems, which can cause flash fires. Instead, heating blocks, water baths, or sand baths should be used.
As scale-up takes place, more care is needed. Increasing temperature speeds up reactions but can also boost the formation of undesirable byproducts. Higher pressure improves the solubility of gases like hydrogen in liquids, but overpressurization increases the risk of an explosion.
Hydrogen storage systems must be well-designed, regularly inspected, and properly maintained to minimize leaks. Effective ventilation in storage areas or enclosures is essential to keep hydrogen levels below the lower flammability limit.
In industrial settings, hydrogen detectors are often placed near reaction vessels and pipelines to alert operators of leaks. Automated shutoff valves, overpressure relief systems, and interlocks tied to emergency protocols provide additional layers of protection. Personnel should also receive regular training in handling flammable gases and catalysts. Following Standard Operating Procedures (SOPs) and wearing proper personal protective equipment (PPE) are non-negotiable.
Despite the near invisibility of hydrogen flames, flame detection equipment can reliably identify them and should always be employed in areas where hydrogen is used or stored.
Conclusion
Hydrogenation reactions are essential to modern life; they contribute to the food we eat, the fuel we burn, and the products we use on our skin, among many others. But with that utility comes risk. Understanding both the intrinsic hazards of hydrogen and the added complexities of hydrogenation chemistry is vital to ensuring safe practices in the lab and in industry.
With thoughtful process design, proper equipment, and robust risk assessments, hydrogenation can continue to play its valuable role—safely and effectively.