Calorimetry: Heat flow versus power compensations methods
Dr. Mario Toubes-Rodrigo
H.E.L Group, 9-10 Capital Business Park, Manor Way, London, WD6 1GW
In this note, we compare the fundamentals and operation of two calorimetry methodologies that the Simular performs: heat flow and power compensation. The mathematical calculations to calculate the energy produced or absorbed by the chemical reaction are presented. The last section directly compares both methods in terms of measurements, temperature control, accuracy, and time.
Introduction
The Simular is a high configurable system designed to optimize process conditions and determining safer reaction conditions. The Simular is designed to operate using the traditional heat flow calorimetry methodology, but also it offers power compensation. The main advantage of this type of calorimetry is the ability to skip time-consuming calibration steps. Glass, stainless steel, and Hastelloy reactor can be used. It operates at temperatures ranging from -30°C (when used with a suitable chiller circulator) to 225°C, and pressures up to 100 barg.
Calorimetry is defined as the science that performs quantitative measurements of heat. It quantifies the transfer of energy between systems caused by temperature differences1. Calorimetry is a very versatile science that has varied applications, such as the calculation of specific heats of solids, liquids, and heat necessary for phase changes. Still, also it can have some fundamental applications in process safety.
Kinetic and thermodynamic characterization of chemical reactions is crucial to understanding the inherent risks of chemical processes. This is where calorimetry plays a fundamental role since most chemical and physical processes involve heat exchange. The heat released or absorbed during a chemical reaction is proportional to the conversion rate (in mol·s-1), and therefore, calorimetry allows for the differential kinetic analysis method2. Knowledge about the behavior and progression of chemical reactions allows for the prediction of hazards and risks, and therefore calorimetry is a fundamental tool to achieve safer processes.
In this technical note, we discuss the basics of the two methodologies that can be used using a H.E.L Simular: heat flow and power compensation.
Heat Flow
Most commercial calorimeters nowadays use heat flow measurements to calculate heat exchange and energy transfer. In this approach, the temperature of the components inside the vessel is controlled by adjusting the fluid inside the jacket. By maintaining isothermal conditions, calorimeters can ensure accurate measurement of heat flow3.
During a chemical reaction in the reactor, energy is generated or absorbed. The heat is then transferred to or from the reaction through the jacket, causing temperature changes. To ensure isothermal conditions, a circulator is used in the calorimeter. The circulator adjusts the temperature of the liquid flowing through the jacket to the desired level, effectively controlling the heat transfer between the jacket and the reactor contents. Precise temperature control in the circulator maintains isothermal conditions within the reactor3.
Calculation using Heat Flow
In this type of calorimetry, the main output signal is the temperature inside the reactor, whereas a thermostat defines the temperature of the jacket. The fluid flow into the jacket is kept high, allowing effective heat exchange with the reactor. The energy balance equation is:
Q = UA(Tr-Tj)
Where U is the effective heat transfer coefficient, A is the surface of heat exchange (or wetted area), Tr is the temperature in the reactor, and Tj is the temperature in the jacket. The main challenge that heat flow measurement face is the potential variation in UA due to dosing and sampling, change in the value due to processes of expansion/contractions, or production of gases as a result of the reaction 2. To solve this problem, calibration steps are necessary before and after the reaction. A heater immersed in the mixture allows for a rectangular heat pulse, resulting in the deviation of temperature in the mix 2. Applying Eq 1:

Knowing the amount of power supplied to the reactor and the initial and final temperatures allows for the calculation of UA. Because of the potential change in volume inside of the reactor during the process, two calibrations would be needed to have representative values of UA, one at the beginning and one at the end.
Power Compensation
In power compensation calorimetry, the temperature of the reaction mixture is kept constant throughout the process. This is achieved by utilizing a calibrated heater inside the vessel. The method compensates for the heat supplied or absorbed by the reaction from the jacket surrounding the reactor, ensuring precise control over the reaction temperature3.
When operating in power compensation, calorimeters operate by fixing the temperature of the oil in the jacket at a defined constant temperature. A calibrated heater inside the reaction vessel is used to regulate the temperature of the reaction mixture. The electrical heater adjusts its power in reaction to the head removal or addition by the jacket, compensating for any heat exchange and ensuring that the temperature inside the vessel is kept constant.
As the chemical reaction progresses, the system releases or absorbs heat. In power compensation calorimetry, the power required to maintain the reaction mixture at a constant temperature is measured directly, and it is equivalent to the heat exchanged by the reaction.
Calculation using Power Compensation
In power compensation, the heater pumps constant power into the reaction to compensate for heat losses, which becomes the baseline. As the reaction progresses, either heat will be generated or absorbed4,5. The systems then will have to adjust to compensate for this energy. In this case:
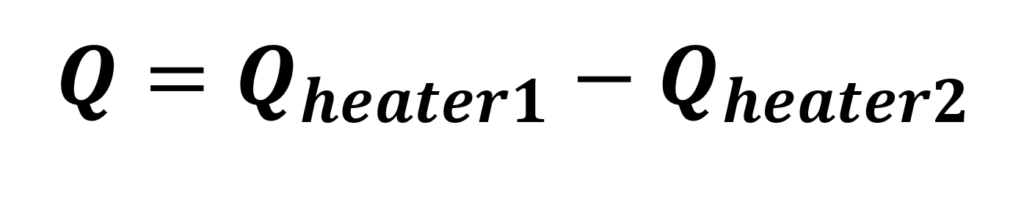
If reaction and heater power are the only terms that vary, temperature changes are restricted to the heater and the thermal boundary layer, which has a small heat capacity. This results in power compensation calorimeters attaining thermal equilibrium more rapidly.
Differences between heat flow and power compensation methodologies
Heat flow and power compensation are commonly used in calorimetry, allowing for the accurate measurement of heat exchange and the quantification of heat flow. However, there are significant differences between both methodologies:
- Measurement approach: in heat flow, the temperature within the reactor is controlled by changing the temperature of the fluid inside the jacket. In power compensation, the power input into the electrical heater determines how much heat is produced or absorbed by the reaction.
- Temperature control: in heat flow, the temperature is adjusted by controlling the jacket’s temperature. In power compensation, the temperature is controlled by the electrical heater.
- Nature of measurement: in heat flow, temperature changes are monitored, whereas, in power compensation, power is the measured variable.
- Accuracy and stability: heat flow calorimeters are very sensitive to small temperature changes, making them suitable for precise heat measurements. Power compensation is highly accurate and stable, as the temperature of the reaction is precisely controlled.
- Time: heat flow requires calibration steps before and after the process, and interpolation of the baseline, while power compensation does not require such steps, speeding up the process.
Conclusion
Heat flow and power compensation methodologies allow for accurate heat exchange and energy transfer measurement. Heat flow relies on accurately measuring temperature changes within the reaction vessel and maintains isothermal conditions to determine heat. On the other hand, power compensation maintains a constant temperature by adjusting the power input to the electrical heater.
References
- Likes, R. Principles of Calorimetric Assay,’in’Passive Nondestructive Assay of Nuclear Materials,’ D. Reilly et al Ed., Los Alamos report LA-UR-90-732. (1991).
- Zogg, A., Stoessel, F., Fischer, U. & Hungerbühler, K. Isothermal reaction calorimetry as a tool for kinetic analysis. Thermochim. Acta (2004).
- Kossoy, A. Reaction calorimetry: Main types, simple theory, and application for kinetic study—A review. Process Saf. Prog. (2023).
- Richner, G., Neuhold, Y.-M. & Hungerbühler, K. Nonisothermal calorimetry for fast thermokinetic reaction analysis: solvent-free esterification of n-butanol by acetic anhydride. Org. Process Res. Dev. 14, 524–536 (2010).
- Guldbæk Karlsen, L. & Villadsen, J. Isothermal reaction calorimeters-I. A literature review. Chem. Eng. Sci. 42, 1153–1164 (1987).




