Discovery
What challenges appear in the discovery phase?
Crystal polymorphism (the precipitation of certain molecules can take different forms, depending on the intermolecular arrangements.1) can result in suboptimal performance. Polymorphs vary in their physico-chemical properties, including solubility and bioavailability, chemical and physical stability, as well as mechanical properties. For this reason, extensive polymorph screening is crucial to identify the optimal structure not only during synthesis but also during use and storage. The complexity of this process and the added lack of knowledge in this field have resulted in the description of crystallization as an art rather than a science.
In silico simulation is used routinely in crystallization to reduce the time and resources used in the discovery phase. However, this methodology also has significant limitations, which can only be addressed by physical testing. Early detection of crystalline forms requires high sensitivity and precision and monitoring multiple parameters simultaneously. This includes close control of factors that impact solubilization processes, such as temperature, and clues about the evolution of the process, such as optical density.
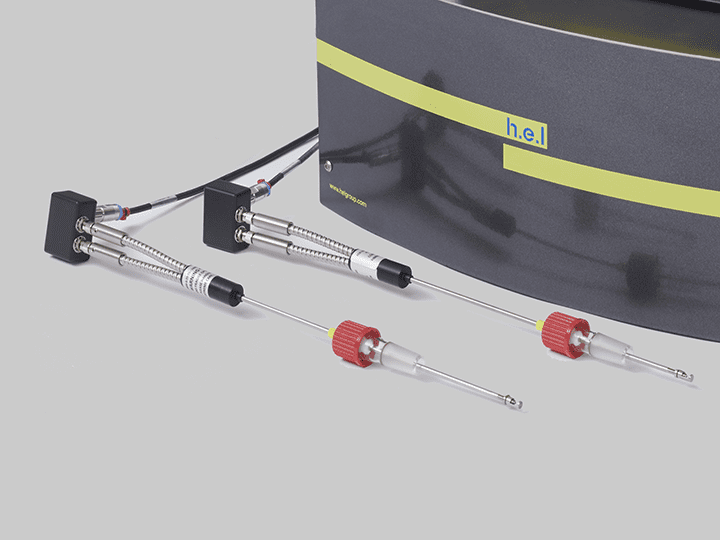
CrystalEYES | Crystallization Monitoring Sensor
Easily Determine Solubility… The need to study crystallization is widespread in the che...
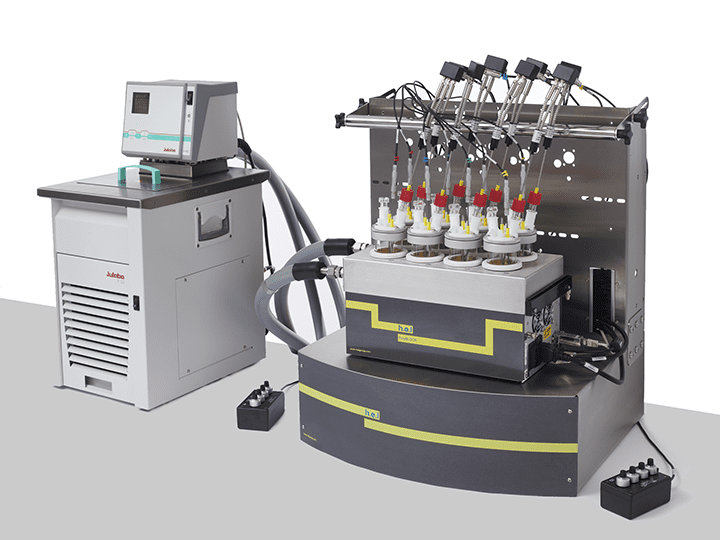
CrystalSCAN | Parallel Crystallization Monitoring Platform
The CrystalSCAN is a bench-top, automated, parallel crystallization monitoring platform, f...
How can reproducibility be increased in the discovery phase?
Crystallization is a highly complex process impacted by numerous factors. Temperature, for instance, significantly affects solubility, impacting both the nucleation and crystal growth processes. Evaporation and cooling, which control the concentration of solute in the mix, are also very heavily influenced by temperature. Minor deviations from the ideal conditions often result in suboptimal outcomes, challenging consistent replication. Automation can significantly enhance reproducibility by minimizing human error and ensuring precise control over experimental conditions2.
Impurities, even in trace amounts, can significantly affect the crystallization process. They may act as nucleation sites, facilitating seed formation and interfering with crystal growth rates, leading to inconsistent results.
Supersaturation is another critical parameter in crystallization, defined as the point where the solute concentration exceeds what is thermodynamically stable. Accurate control and measure of supersaturation are essential for controlling nucleation rates and ensuring consistent crystal formation.
Solutions
CrystalEYES is H.E.L’s crystallization monitoring sensor, which can detect changes in the solution’s turbidity that indicate the precipitation processes. CrystalEYES provides insights into the process, allowing parameter adjustments to optimize conditions to increase the reproducibility and stability of the desired crystal form.
CrystalSCAN is designed for parallel crystallization monitoring and significantly accelerating the screening of parameters in the discovery phase. This is a powerful tool for identifying the optimal nucleation and crystal growth conditions. CrystalSCAN’s ability to determine solubility curves of metastable zone widths makes it a fantastic instrument for understanding the impact of physicochemical variables in the process.

CrystalEYES | Crystallization Monitoring Sensor
Easily Determine Solubility… The need to study crystallization is widespread in the che...

CrystalSCAN | Parallel Crystallization Monitoring Platform
The CrystalSCAN is a bench-top, automated, parallel crystallization monitoring platform, f...
Development
What are the challenges in optimizing conditions for crystal growth and purity during development?
The main driving force in the crystallization process is the solubility of the compound of interest. The solubility depends on a range of variables:
- Solvent(s): due to the interaction with the solutes, solvents can stabilize or destabilize different crystal forms. For instance, solvents that strongly interest the molecule of interest can enhance the solubility of the crystal by interfering with the intermolecular bonds within the solute and vice versa. In multi-solvent systems, the solvent ratio can drastically affect the polarity of the medium and consequently affect the solubility, leading to different crystal forms.
- Temperature: although an increase in temperature generally tends to favor the solubility of solids in liquid, this is not universal. Temperature changes also influence the nucleation and growth rate of crystals. Thermodynamics dictates that at higher temperatures, the molecular motion increases, which, in turn, can drastically affect crystal quality and size distribution.
- pH: the pH of a solution will impact the charge state of solute molecules, especially in the case of ionic compounds or molecules that contain ionizable groups. Changes in the ionic state will affect the solubility of the molecule. Additionally, pH can affect nucleation processes due to its influence on the electrostatic interaction between atoms and molecules.

CrystalEYES | Crystallization Monitoring Sensor
Easily Determine Solubility… The need to study crystallization is widespread in the che...

CrystalSCAN | Parallel Crystallization Monitoring Platform
The CrystalSCAN is a bench-top, automated, parallel crystallization monitoring platform, f...
How can efficiency be increased in this phase?
While significant efforts have been directed toward increasing the throughput of the screening phase, the development phase has not received the same level of attention. In the screening phase, as previously discussed, the primary focus was identifying physical and chemical conditions favorable for crystal formation. However, the focus shifts on this second phase towards refining these conditions to optimize crystal production3,4.
To increase the efficiency of this phase, it is crucial to design matrixes that encompass the conditions that will impact nucleation and crystal growth. This can include different solutions, solvents, pH levels, and temperatures. To make it effective, these designed arrays must systematically modify the physicochemical variables to fine-tune the crystallization environment. Some approaches start with broad, coarse rangers that are adjusted more granularly and precisely until the desired crystal volume and quality are achieved.3
By methodically refining these variables, researchers can develop optimized crystallization processes that yield higher volumes of suitable crystals, thereby enhancing the overall productivity and efficacy of the development phase.
Solutions
While significant efforts have been directed toward increasing the throughput of the screening phase, the development phase has not received the same level of attention. In the screening phase, as previously discussed, the primary focus was identifying physical and chemical conditions favorable for crystal formation. However, the focus shifts on this second phase towards refining these conditions to optimize crystal production3,4.
To increase the efficiency of this phase, it is crucial to design matrixes that encompass the conditions that will impact nucleation and crystal growth. This can include different solutions, solvents, pH levels, and temperatures. To make it effective, these designed arrays must systematically modify the physicochemical variables to fine-tune the crystallization environment. Some approaches start with broad, coarse rangers that are adjusted more granularly and precisely until the desired crystal volume and quality are achieved.3
By methodically refining these variables, researchers can develop optimized crystallization processes that yield higher volumes of suitable crystals, thereby enhancing the overall productivity and efficacy of the development phase.

CrystalEYES | Crystallization Monitoring Sensor
Easily Determine Solubility… The need to study crystallization is widespread in the che...

CrystalSCAN | Parallel Crystallization Monitoring Platform
The CrystalSCAN is a bench-top, automated, parallel crystallization monitoring platform, f...
Scale-Up
What issues arise during scale-up?
The scale-up process often faces challenges rooted in mixing and resultant complications, such as heat transfer, among others. As production moves from bench top towards industrial scale, the homogeneity achieved through mixing becomes more challenging to maintain. This can lead to uneven temperature and solute concentration distribution, which can be detrimental to the nucleation and crystal growth phases. This variability can result in non-uniform particle size distribution and shape anomalies, compromising quality and yield. The change in scale also typically affects heat transfer efficiency, as large volumes add an extra layer of difficulty for how the heat needs to be distributed or removed, complicating the maintenance of the precise temperature required for optimal crystallization.
Other factors impacted by scale-up include fluid dynamics and suspension behavior. This can affect, for example, the frequency at which fresh superheated solution is delivered to critical zones. This can drastically affect the supersaturation levels. These changes require meticulous testing to recalibrate these factors and be able to replicate the conditions achieved on smaller scales. Without careful adjustment and optimization of these factors, scale-up efforts can lead to increased production costs, extended development times, and even failure to meet regulatory and quality standards, posing substantial risks to commercial viability.
What challenges are encountered at a large scale?
In scaling up crystallization to commercial production, the push to maximize efficiency and reduce costs often can lead to variability that can significantly affect the quality and safety of the final product. Large-scale operations may struggle to maintain the precise and controlled conditions required to obtain optimal products, resulting in variations in the physical properties of the crystals. Such alterations not only impact the effectiveness of the products but also result in safety risks – especially when dealing with hazardous or explosive substances 6.
Companies must address these challenges by performing accurate and realistic risk assessments, utilizing robust quality systems, and using advanced monitoring techniques. This proactive approach helps to maintain consistency across batches without compromising health and safety. It is fundamental that the reactors and crystallizers are manufactured with durable and appropriate materials, mitigating the introduction of impurities and preventing equipment degradation. By integrating these strategies, manufacturers can safeguard both the integrity of their products and the health and safety of their operations, ensuring they meet both commercial demands and regulatory standards.
Solutions
In scaling up crystallization to commercial production, the push to maximize efficiency and reduce costs often can lead to variability that can significantly affect the quality and safety of the final product. Large-scale operations may struggle to maintain the precise and controlled conditions required to obtain optimal products, resulting in variations in the physical properties of the crystals. Such alterations not only impact the effectiveness of the products but also result in safety risks – especially when dealing with hazardous or explosive substances 6.
Companies must address these challenges by performing accurate and realistic risk assessments, utilizing robust quality systems, and using advanced monitoring techniques. This proactive approach helps to maintain consistency across batches without compromising health and safety. It is fundamental that the reactors and crystallizers are manufactured with durable and appropriate materials, mitigating the introduction of impurities and preventing equipment degradation. By integrating these strategies, manufacturers can safeguard both the integrity of their products and the health and safety of their operations, ensuring they meet both commercial demands and regulatory standards.

CrystalSCAN | Parallel Crystallization Monitoring Platform
The CrystalSCAN is a bench-top, automated, parallel crystallization monitoring platform, f...
References
1. Ajito, K., Nakamura, M., Tajima, T. & Ueno, Y. Terahertz spectroscopy methods and instrumentation. (2017).
2. Thakur, A. S. et al. Improved success of sparse matrix protein crystallization screening with heterogeneous nucleating agents. PLoS One 2, e1091 (2007).
3. Luft, J. R. et al. Efficient optimization of crystallization conditions by manipulation of drop volume ratio and temperature. Protein Sci. 16, 715–722 (2007).
4. Chayen, N. E. & Saridakis, E. Protein crystallization for genomics: towards high-throughput optimization techniques. Acta Crystallogr. D Biol. Crystallogr. 58, 921–927 (2002).
5. Nere, N. K. et al. CASE STUDIES ON CRYSTALLIZATION SCALE-UP. Chem. Eng. Pharm. Ind. Act. Pharm. Ingred. 617–633 (2019).
6. Ettouney, R. & El-Rifai, M. Explosion of ammonium nitrate solutions, two case studies. Process Saf. Environ. Prot. 90, 1–7 (2012).


