Integration of mass spectrometry to characterize the gas evolution of batteries in thermal runaways using adiabatic caolorimetry.
Dr. Mario Toubes-Rodrigo, Dr. William Yao and Carl Chen
H.E.L Group, Unit 2 Centro, Boundary Way, Hemel Hempstead, HP2 7SU, UK
Table of Contents
Abstract
Lithium-ion batteries are an essential energy storage technology, but their increasing energy density raises significant safety concerns. Reports of thermal runaway incidents highlight the urgent need for improved methods to study and mitigate these hazardous events. A key challenge in battery safety research is understanding the gas evolution during thermal runaway, as conventional techniques such as gas chromatography operate under conditions that do not fully replicate high-pressure, high-temperature environments. Here, we present a novel integration of adiabatic calorimetry (BTC-500) with high-pressure mass spectrometry to characterize gas evolution during lithium-ion battery thermal runaway. This approach enables real-time, high-frequency gas composition analysis under extreme conditions, overcoming limitations of conventional gas analysis techniques. Compared to standard gas chromatography, which requires sample
transfer and offline analysis, in-situ mass spectrometry provides a direct and dynamic measurement of evolving gases. This allows for a more comprehensive understanding of reaction pathways, including the detection of transient species that may otherwise be missed. The study identified key differences in gas compositions between two battery types, highlighting the variability in decomposition reactions and gas evolution mechanisms. By providing real-time insights into the chemical processes occurring during thermal runaway, this method advances battery safety research. A better understanding of gas formation dynamics can inform the design of safer battery materials and improve predictive models for failure scenarios. These findings demonstrate how high-pressure mass spectrometry, when coupled with adiabatic calorimetry, can enhance safety assessments and accelerate the development of next-generation lithium-ion batteries.
Introduction
The increasing global demand for more powerful and energy-dense lithium-ion batteries has brought battery safety to the forefront of research. Reports of fire and explosions have become commonplace in the news, emphasizing the need for improved safety measures.
These fires are a result of thermal runaways, a process in which exothermic reactions within the battery enter a feedback loop. The accumulation of heat within the battery triggers a series of decomposition reactions that release heat and gases. The gases produced from the decomposition can be flammable, depending on the nature of the electrolyte. Oxygen can be released when the metal oxides that form the cathode, combined with the flammable gases and heat, can create ideal conditions for catastrophic fires and explosions.
As the temperature of a lithium-ion battery increases, a series of progressively complex reactions occur. At temperatures above 70°C, lithium salts decompose and react with solvents, forming a new Solid Electrolyte Interphase (SEI). Between 90-130°C, the SEI layer ((CH₂OCO₂Li)₂) decomposes, releasing heat and gases like C₂H₄, CO₂, and O₂, while the anode surface loses SEI protection at around 120°C, allowing embedded lithium to react with electrolyte solvents (e.g., ethylene carbonate, propylene carbonate), producing CxHy gases. Electrolyte decomposition begins around 110°C and continues up to 230°C, releasing fluoride compounds. Simultaneously, the separator shrinks and melts between 130-190°C, weakening structural integrity. At 200-300°C, cathode materials decompose, releasing O₂, with onset temperatures varying by material—e.g., ~150°C for LiCoO₂, ~220°C for NCM811, and ~310°C for LiFePO₄. Beyond 230°C, the binder polyvinylidene fluoride (PVDF) reacts with lithium and decomposes. By 235°C, thermal runaway occurs, producing gases such as CO₂, CO, H₂, CxHy, CxHyOz, CxHyF, POF₃, and HF, while the vaporized electrolyte adds further volatile components, intensifying the hazard.
These reactions do not occur strictly within the specified temperature ranges; as the temperature increases, the reactions become progressively more complex and intertwined.
H.E.L Group’s BTC-500 is a benchtop adiabatic calorimeter designed to enable a comprehensive analysis of batteries’ thermal behavior. It evaluates thermal, electrical, and mechanical stress responses, providing information on the battery’s safe operating limits.
The aim of this Application Note is to study the gas evolution profile of two batteries using a novel combination of adiabatic calorimetry (BTC-500) and gas analysis using in-situ mass spectroscopy.
Material and methods
Lithium-ion batteries
Two LiFP batteries, one with a capacity of 151 Ah and one with a capacity of 177 Ah, were used in this experiment. Both were at 100% SOC prior to the experiment.
Adiabatic Calorimetry (BTC-500) equipped with High-Pressure Mass Spectrometry
A Mass Spectrometer was attached to a BTC-500 (H.E.L Group, Hemel Hempstead, UK) in a way the gases produced during the LIB thermal runaway could be sampled. This allowed for the analysis of gas with very high-frequency data acquisition (50 ms per mass number) at high pressure (8 bar) and high temperature.

Figure 1, BTC-130 attached to mass spectrometer
Batteries were subjected to thermal runaway using Heat-Wait-Search plan. When the battery entered the thermal event, an in-situ online analysis was performed, providing real-time monitoring of the chemical reactions that were occurring. Periodic online sampling was conducted, taking
samples at predefined intervals during the reaction. Additionally, gas samples were collected after the thermal runaway was completed, allowing the retrospective analysis of transient reactions that might have been missed.
Results and Discussion
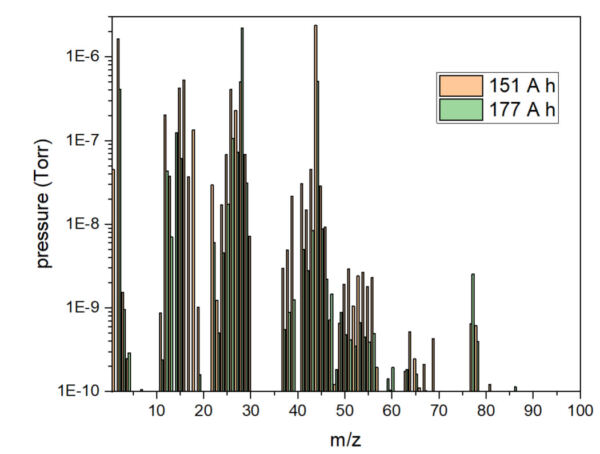
Figure 2. The composition of the gas produced by two batteries (151 A h and 177 A h) during thermal runaway in a BTC-500.
Figure 2 shows the gas produced by two different types of battery. After the thermal runaway of a 151 Ah cell, a large amount of CO₂, H₂, CH₄, C₂H₄, CO, and small amounts of SO₂ and mercaptamines were detected. After the thermal runaway of a 177Ah cell, a large amount of CO, CO₂, H₂, and small amounts of toluene, trimethylamine N-oxide, and long-chain hydrocarbons were identified.
Limitations of Conventional Methods: Currently, gas chromatography (GC) is the most used method for gas analysis. However, GC requires analysis at relatively low pressures and temperatures, making it unsuitable for evaluating gas compositions under high-temperature and high-pressure conditions.
Advantages of In-Situ Online Analysis: The in-situ method allows gas analysis enables the measurement pre-, during, and post thermal runaway, reflecting the chemical states and reaction processes in real-time. This approach provides a more comprehensive understanding of the various chemical reactions occurring during each stage of a thermal runaway.
This collaboration has introduced a high value feature through high-pressure mass spectrometry (MS) integration with H.E.L’s BTC series. Enabling high-frequency data analysis at 50 ms per mass unit and offering gas analysis under high-pressure and temperature conditions.
Conclusion
Thermal runaway is a key focus in lithium-ion battery safety research. H.E.L BTC-500 Adiabatic Accelerating Rate Calorimeter equipped with an in-situ mass spectrometer (MS) enabled real-time, high-frequency gas composition analysis under extreme conditions of high pressure and temperature. Enhancing engineers and researchers ability to monitor and study gas evolution during lithium-ion battery thermal runaway events.



