Identifying the best rhodium-modified catalyst and the best reaction conditions with ChemSCAN for the synthesis of aroma compounds
Dilver Peña Fuentes1, Mario Toubes-Rodrigo2
1 Leibniz Institute for Catalysis, Albert-Einstein-Str. 29a, 18059 Rostock, Germany
2 H.E.L Group, Unit 2 Centro, Boundary Way, Hemel Hempstead, HP2 7SU, UK
Abstract
In the perfumery industry, there is a constant need to provide compounds imparting novel organoleptic notes. In this study, a ChemSCAN is used to investigate the hydroformylation of the substrate 4,4-dimethyl-1-vinylcyclohexyl acetate as a precursor for the desired 3-(cyclohex-1-en-1-yl)propanal derivative. Fast parallel trials allowed for the identification of the most active and selective modified rhodium catalyst. Subsequent concurrent experiments enabled the evaluation of different catalyst loadings and minimized the amount of solvent employed.
Table of Contents
Introduction
In the perfumery industry, there is a constant need to provide compounds imparting novel organoleptic notes. Aldehydic notes, which represent one of the key organoleptic facets of the lily of the valley odor are of particular interest. 3-(cyclohex-1-en-1-yl)propanal derivatives represent compounds imparting note of the muguet-aldehydic olfactive family. However, access to these derivatives is cumbersome and requires Grignard reagents, radical chemistry, or pyrolysis, to deliver the desired compounds with low yield and/or selectivity.[1]
The selective hydroformylation of 4,4-dimethyl-1-vinylcyclohexyl acetate could provide the desired 3-(cyclohex-1-en-1-yl)propanal derivative. During this study the hydroformylation of 4,4-dimethyl-1-vinylcyclohexyl acetate was investigated using a ChemSCAN to identify the most appropriate rhodium catalyst and best reaction conditions. Different ligands were evaluated in fast parallel experiments, allowing the selection of the most active and selective catalyst. Subsequent concurrent experiments enabled to find the best reaction conditions for the appropriate scalable synthesis of the desired fragrance.
H.E.L’s ChemSCAN is a bench-top advanced parallel screening platform, designed to accelerate process development. Controlled by H.E.L’s WinISO, it enables fully automated, high-throughput investigations. ChemSCAN allows researchers to efficiently investigate reactions conditions, and optimize processes with great precision and speed. By offering precise control over variables such as temperature, pressure, and mixing, ChemSCAN enables reproducible results and enhances overall productivity in chemical research and development.
The aim of this study was the identification of the most suitable rhodium catalysis and the ideal conditions using H.E.L’s ChemSCAN capabilities.
Materials and Method
For the catalytic test, a ChemSCAN was used within a fume hood. The substrate 4,4-dimethyl-1-vinylcyclohexyl acetate was provided by Firmenich International SA. Precatalysts and ligands solutions were prepared under an argon atmosphere using standard Schlenk techniques. The solvents were dried by conventional procedures and distilled under an argon atmosphere. The structure of products was confirmed by NMR measurements on Bruker AV 300, AV 400, or AV 500 MHz spectrometers. Gas chromatography was performed on an Agilent 7890 A Series equipped with an HP5 column (30 m x 0.25 mm ID, 0.25µm film) using tetradecane as the internal standard.
General hydroformylation procedure:
4,4-dimethyl-1-vinylcyclohexyl acetate, Ligand (in EtOAc) and Rh(acac)(CO)2 (in EtOAc) were added to an autoclave (H.E.L. ChemSCAN). The autoclave was purged 3 times with 8 bar Argon and 4 times with 10 bar syngas (H2:CO, 1:1) under stirring (500 rpm). The autoclave was then charged with 10 bar syngas and the reaction mixture was heated until the temperature raised to 5°C under the target value. The autoclave was then further pressurized with syngas to the target pressure, the stirring rate adjusted at 900 rpm and the temperature was set to final value. The hydroformylation was continued compensating the gas uptake with H2:CO (1:1). After the indicated reaction time, the mixture was cooled to room temperature, the pressure released, and the autoclave was purged 5 times with 12 bar Argon. Product analysis was performed by gas chromatography using tetradecane as internal standard.
Results and Discussion
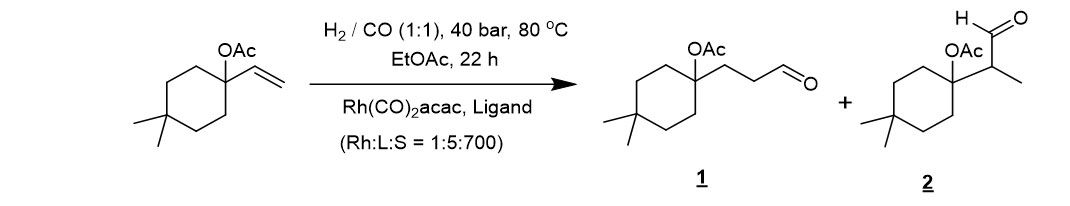 Scheme 1. Hydroformylation of 4,4-dimethyl-1-vinylcyclohexyl acetate.
Scheme 1. Hydroformylation of 4,4-dimethyl-1-vinylcyclohexyl acetate.
The hydroformylation of 4,4-dimethyl-1-vinylcyclohexyl acetate was evaluated by parallel screening of several ligands (Scheme 1). Some results of the reaction outcome using different ligands are shown in Table 1. Full conversion and excellent selectivity were achieved when bidentate diphosphite ligand BiPhePhos was used. The rhodium-modified catalyst with bidentate diphosphine Xantphos-type ligands showed lower activity but still good selectivity towards the desired n-Aldehyde 1. Other more flexible bidentate diphosphine ligands like BISBI and DPEphos did not show a good performance.
Table 1: Hydroformylation of 4,4-dimethyl-1-vinylcyclohexyl acetate catalyzed by rhodium complexes with different ligands.
| Nr. | Ligand | L/Rh | Conversion 1) | Selectivity 1) | Yield 1) |
|---|---|---|---|---|---|
| 1 | Xantphos | 5 | 41% | 99/1 | 41% |
| 2 | Nixantphos | 5 | 46% | 99/1 | 46% |
| 3 | BISBI | 5 | 32% | 86/7 | 28% |
| 4 | BiPhePhos | 5 | 100% | 98/0.3 | 98% |
| 5 | DPEphos | 5 | 30% | 92/5 | 28% |
1) Determined by GC analysis
The Rh-catalyzed hydroformylation of 4,4-dimethyl-1-vinylcyclohexyl acetate with bidentate diphosphite ligand BiPhePhos was further optimized in terms of the concentration of reactants and catalyst loading by parallel screening reactions. Initially, the catalyst loading could be reduced to 0.05 mol% Rh while dropping the amount of solvent without affecting the n-selectivity and reaching full conversion in only 3.5 h (Table 2, entry 5). Further decrease of the catalyst loading while increasing the concentration of the reactants to the practical minimum amount of solvent needed showed that the hydroformylation reaction proceeded with almost full conversion and excellent selectivity even with a very low catalyst loading of 1.25 x 10-3 mol% Rh (Table 2, entry 14).
| Nr. | Rh:L:S | V(solvent) | Reaction | Conversion 1) | Selectivity1)
(1/2) |
Yield 1)
(1) |
|---|---|---|---|---|---|---|
| 1 | 1:5:700 | 14 mL | 22 h | 100% | 98/0.3 | 98% |
| 2 | 1:5:700 | 9.6 mL | 7 h | 100% | 97/0.3 | 97% |
| 3 | 1:5:2 000 | 9.6 mL | 18 h | 99% | 98/0.2 | 97% |
| 4 | 1:5:2 000 | 4.0 mL | 18 h | 100% | 97/0.1 | 97% |
| 5 | 1:5:2 000 | 1.2 mL | 3.5 h | 100% | 96/0.1 | 96% |
| 6 | 1:5:4 000 | 1.2 mL | 12 h | 98% | >99/0.1 | 97% |
| 7 | 1:5:8 000 | 1.2 mL | 12 h | 97% | >99/0.2 | >96% |
| 8 | 1:5:20 000 | 1.2 mL | 22 h | 97% | 94/0.2 | 91% |
| 9 | 1:2.5:20 000 | 1.2 mL | 22 h | 96% | 98/0.2 | 94% |
| 10 | 1:2.5:20 000 | 0.4 mL | 22 h | 97% | >99/0.2 | >96% |
| 11 | 1:2:40 000 | 0.4 mL | 22 h | 97% | 97/0.2 | 94% |
| 12 | 1:2:80 000 | 0.4 mL | 22 h | 87% | 98/0.2 | 85% |
| 13 | 1:2:80 000 | 0.2 mL | 22 h | 86% | 99/0.2 | 85% |
| 14 | 1:2:80 000 | 0.2 mL | 36 h | 97% | >99/- | >96% |
1) Determined by GC analysis
Conclusion
The optimization experiments for the hydroformylation of 4,4-dimethyl-1-vinylcyclohexyl acetate with the parallel reactor system. H.E.L. ChemSCAN showed that it is possible to carry out the reaction with a catalyst loading of 1.25 x 10-3 mol% in 36 h, achieving almost full conversion (97%) and perfect regioselectivity. Thus, the quick parallel experiments enabled to find the best reaction conditions for the appropriate scalable synthesis of the desired fragrance.[1]
References
[1].KNOPFF, O.; DUPAU, P.; RIEDHAUSER, J-J.; POIRIER, N.; PEÑA FUENTES, D.; BÖRNER, A.; MARINONI, L. (to Firmenich International SA) WO Patent 2022049036-A2, 2022. Novel process for the preparation of 3-(cyclohex-1-en-1-yl)propanal for producing perfume ingredient derivatives, 2022.




