Investigating Hydrogenations at Different Catalyst Loadings using the ChemSCAN
Dr. Sina Ehsani, Joseph Willmot
H.E.L Group, 9-10 Capital Business Park, Manor Way, London, WD6 1GW
In this study, a ChemSCAN is used to investigate the hydrogenation of nitrobenzene with a range of Pd/C catalyst loadings ranging from 4.0 mg to 9.3 mg. The experimental results showed that the hydrogenation reaction rate increases as the amount of catalyst in the reactor increases. The total volumetric uptake of this reaction was not affected by the amount of catalyst in the reactor and was in good agreement with the theoretical values. The results highlight how a ChemSCAN can be used for other similar catalytic studies.
Table of Contents
Introduction
The ChemSCAN is a bench-top, automated parallel catalyst screening reactor system. It was designed for the rapid screening of reactions and catalysts at high pressures. The eight-reactor variant supports reactors ranging from 16 ml to 50 ml. The four-reactor variant can support direct agitation and reactors up to 500 ml. The independently controlled reaction zones are beneficial for design of experiment (DoE) studies and are found in other products such as the BioXplorer and PolyBLOCK 8. Typically, stainless steel (SS316) or hastelloy (HC276) reactors are used. The ChemSCAN operates at temperatures ranging from -40 ˚C (when used with a suitable chiller circulator) to 250 ˚C, and pressures up to 200 bargs. Each stirred reactor is independently controlled and monitored, allowing screening tests to be carried out concurrently, accelerating development time.
Aniline is a primary aromatic amine commonly used in the manufacture of polymers, rubber, agricultural chemicals, dyes and pigments, pharmaceuticals, and photographic chemicals [1]. The hydrogenation of nitrobenzene using precious metal catalysts such as copper (Cu), palladium (Pd), and platinum (Pt) is an important aniline synthesis route [2]. Palladium-supported catalysts are one of the most important groups of heterogenous catalysts, widely used in hydrogenation reactions [3]. Catalyst supports enhance the efficiency of the supported metals by acting as a catalytically active center [4]. Due to their large surface area and low intrinsic chemical activity, activated carbons are frequently used as supports for noble metals [5]. The activated Pd/C materials are used as efficient catalysts for the hydrogenation of nitrobenzene [6].
In this study, the ChemSCAN was used to measure the volumetric uptake of hydrogen gas (H2) during the hydrogenation reaction of nitrobenzene over a catalyst (palladium supported on activated carbon, Pd/C) loading range of 4.0 mg to 9.3 mg. The measured volumetric uptakes obtained from the ChemSCAN for each of the reactors were compared to the theoretical values.
Materials and Method
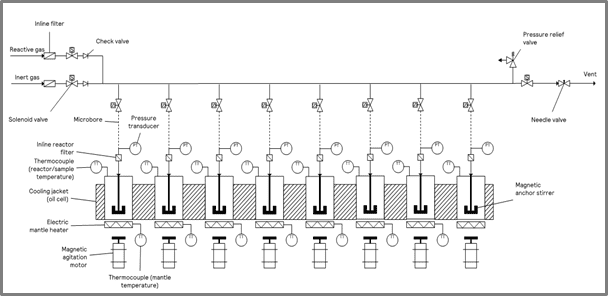
For this characterization, a ChemSCAN was used under standard laboratory conditions within a fume hood. A schematic of the ChemSCAN with eight reactor zones is shown in Figure 1.
This study aimed to investigate the catalytic hydrogenation of nitrobenzene (Sigma-Aldrich Code: N1,095-0) using 1% Palladium supported on activated carbon (Sigma-Aldrich Code: 20,567-2), under various catalyst loading levels. 1-Decanol (Sigma-Aldrich Code: 15,058-4) was selected as the solvent due to its low vapor pressure, high boiling point, and miscibility with nitrobenzene.
A solution of 4.75 mass% nitrobenzene was prepared by mixing 20.0 grams of nitrobenzene in 401.1 grams of 1-decanol. The required amount of the sample solution (nitrobenzene-decanol) along with the desired amount of catalyst (Pd/C) were added to each of the reactor vessels.
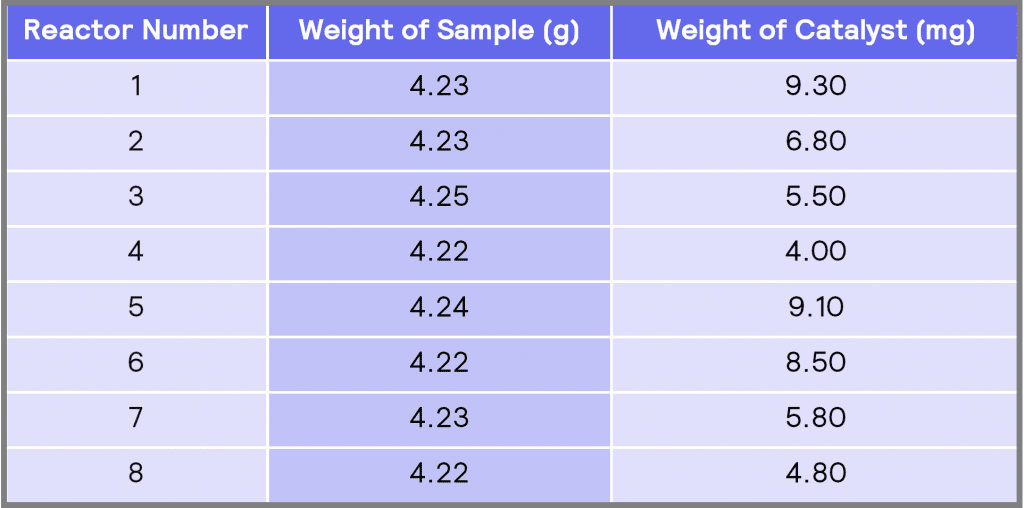
Table 1: The amount of sample (nitrobenzene-decanol) and catalyst (Pd/C) used in each reactor
Table 1 shows the weight of the sample and the weight of the catalyst add to each of the reactors. All these reactions were performed at a constant temperature of 30 ˚C and a constant agitation speed of 500 rpm. A schematic of the 16 mL vessels is shown in Figure 2. The full experimental procedure is described in Appendix B.
 |
 |
| Figure 1: A schematic of HP ChemSCAN with 8 reactor zones. | Figure 2: A schematic of the 16 mL stainless-steel reactor vessel. |
Results and Discussion
The results of the hydrogenation experiments at various catalyst loadings (ranging from 4.0 mg to 9.3 mg) are shown in Figure 3. It should be noted that all volumetric uptake values, regardless of the amount of catalyst used, are within a maximum variation of 4.4 % of theoretical values. This demonstrates that the amount of catalyst only affected the reaction rate, not the final reaction or overall volumetric uptake.
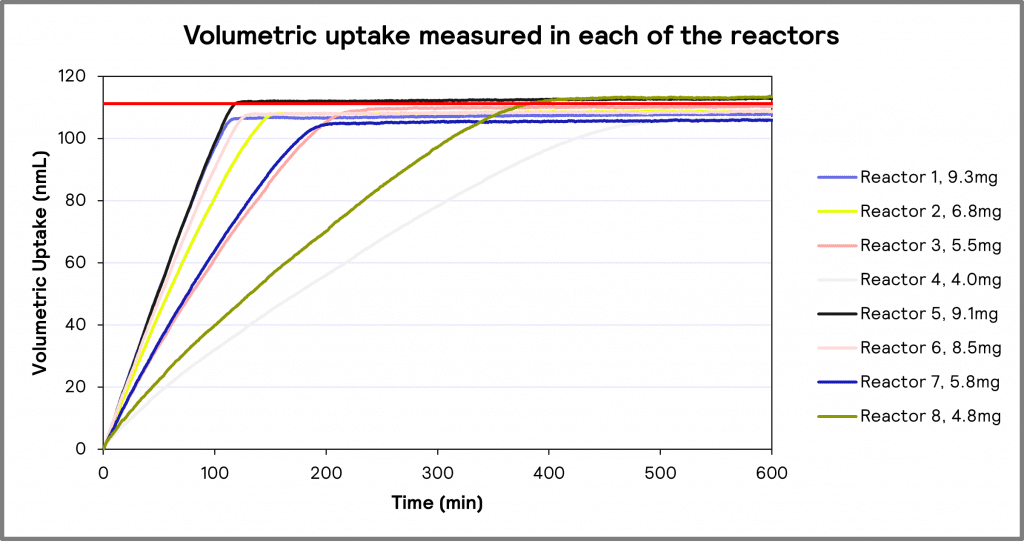
Figure 3: Volumetric uptake measured in each of the reactors.
Figure 3 also demonstrates that as catalyst loading increases, the rate of the hydrogenation reaction increases. That is, a catalyst loading of 9.3mg would result in the fastest hydrogenation process, which would be expected.
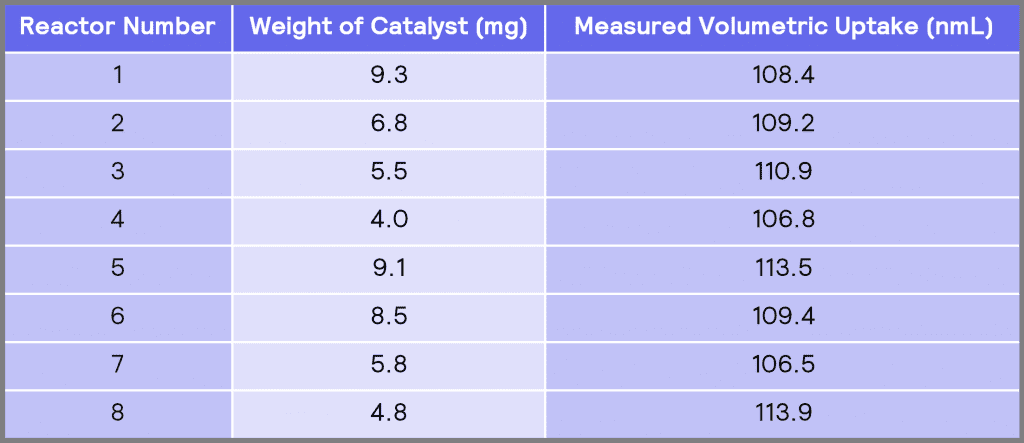
Table 2: Measured volumetric uptake in each of the reactors.
Table 2 shows the measured volumetric uptake in each of the reactors. It can be seen that the total volumetric uptakes in different reactors are similar to each other, indicating the degree of measurement precision obtained using the ChemSCAN. The measured volumetric uptakes have a mean value of 109.8 nmL and a standard deviation (σ) of 2.8 nmL. Considering there is approximately 0.20g of nitrobenzene in each reactor, the theoretical volumetric uptake in each reactor is 111.2 nmL (Appendix A – Sample Calculations). Therefore, the average measured volumetric uptake obtained using HP ChemSCAN is 98.8% of the theoretical value.
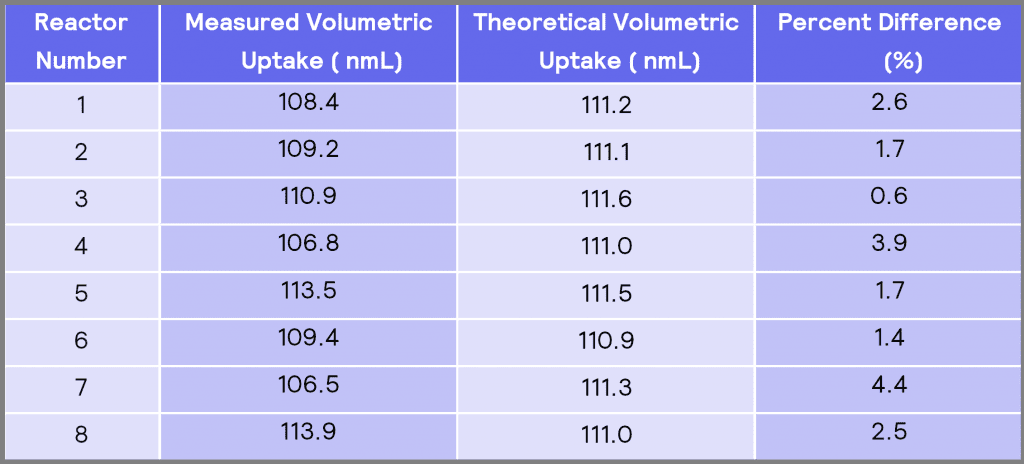
Table 3: A comparison between the measured volumetric uptake and the theoretical volumetric uptake.
Table 3 compares the measured volumetric uptake in each reactor to the theoretical (normalized) volumetric uptake. The results in Table 3 show that the minimum difference between the measured volumetric uptake and the theoretical volumetric uptake is obtained in reactor #3 of 0.6 %. In contrast, reactor #7 has the maximum difference of 4.4% to theoretical values.
Conclusions
The purpose of this study was to investigate the hydrogenation reaction of nitrobenzene with different catalyst loadings using the ChemSCAN. Different amounts of the catalyst, palladium supported on activated carbon, were added to each of the eight reactors, and the volumetric uptakes were measured using the ChemSCAN. These experiments demonstrated that an increase in the amount of catalyst inside the reactor results in a faster hydrogen gas (H2) uptake, presumable due to a quicker hydrogenation reaction.
In addition, the results demonstrated that the total hydrogen volumetric uptake is not affected by the amount of catalyst inside the reactor. Rather, the amount of the catalyst inside the reactor would only affect the reaction kinetics.
A comparison between the measured volumetric uptakes using the ChemSCAN and the theoretical uptakes calculated using the ideal gas law demonstrated that the ChemSCAN can be used to accurately characterize hydrogenation reactions. This is essential information when screening catalysts, as the ChemSCAN can easily and readily differentiate the performance of each set of reaction conditions. For example, defining optimal reaction conditions by keeping the chemistry the same and changing the reaction parameters in a DoE study.
References
1. Amini, B. and S. Lowenkron, Aniline and its derivatives. Kirk‐Othmer Encyclopedia of Chemical Technology, 2000.
2. Boymans, E.H., P. Witte, and D. Vogt, A study on the selective hydrogenation of nitroaromatics to N-arylhydroxylamines using a supported Pt nanoparticle catalyst. Catalysis Science & Technology, 2015. 5(1): p. 176-183.
3. Mironenko, R.M., O.B. Belskaya, and V.A. Likholobov, Approaches to the synthesis of Pd/C catalysts with controllable activity and selectivity in hydrogenation reactions. Catalysis Today, 2020. 357: p. 152-165.
4. Shinde, P.S., et al., A Brief Overview of Recent Progress in Porous Silica as Catalyst Supports. Journal of Composites Science, 2021. 5(3): p. 75.
5. Suh, D.J., T.J. Park, and S.K. Ihm, Characteristics of carbon-supported palladium catalysts for liquid-phase hydrogenation of nitroaromatics. Industrial & engineering chemistry research, 1992. 31(8): p. 1849-1856.
6. Willocq, C., et al., Hydrogenation of nitrobenzene over Pd/C catalysts prepared from molecular carbonyl–phosphine palladium clusters. Journal of Molecular Catalysis A: Chemical, 2012. 365: p. 172-180





