Development
What are the challenges in optimizing conditions for crystal growth and purity during development?
The main driving force in the crystallization process is the solubility of the compound of interest. The solubility depends on a range of variables:
- Solvent(s): due to the interaction with the solutes, solvents can stabilize or destabilize different crystal forms. For instance, solvents that strongly interest the molecule of interest can enhance the solubility of the crystal by interfering with the intermolecular bonds within the solute and vice versa. In multi-solvent systems, the solvent ratio can drastically affect the polarity of the medium and consequently affect the solubility, leading to different crystal forms.
- Temperature: although an increase in temperature generally tends to favor the solubility of solids in liquid, this is not universal. Temperature changes also influence the nucleation and growth rate of crystals. Thermodynamics dictates that at higher temperatures, the molecular motion increases, which, in turn, can drastically affect crystal quality and size distribution.
- pH: the pH of a solution will impact the charge state of solute molecules, especially in the case of ionic compounds or molecules that contain ionizable groups. Changes in the ionic state will affect the solubility of the molecule. Additionally, pH can affect nucleation processes due to its influence on the electrostatic interaction between atoms and molecules.
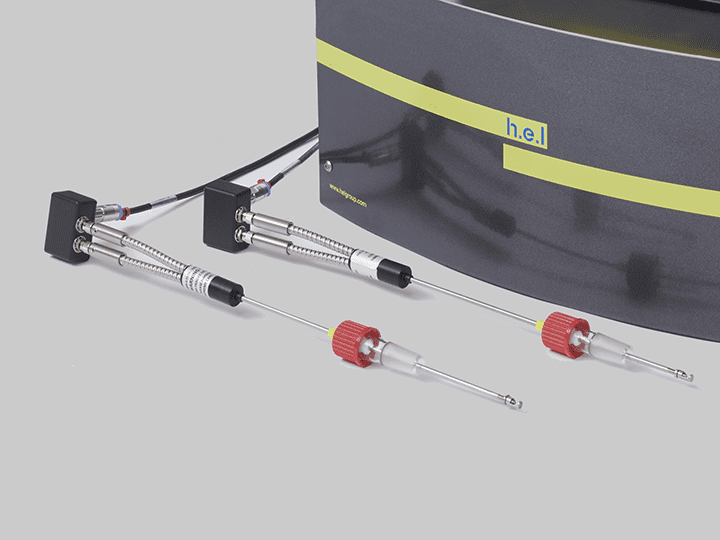
CrystalEYES | Crystallization Monitoring Sensor
Easily Determine Solubility… The need to study crystallization is widespread in the che...
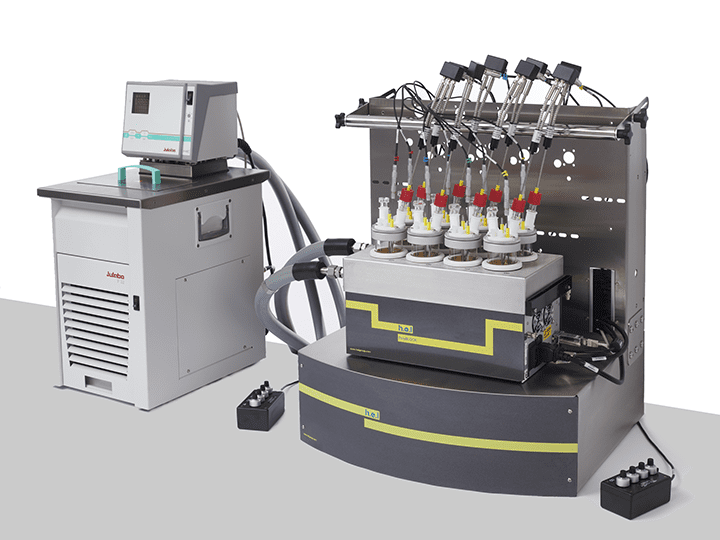
CrystalSCAN | Parallel Crystallization Monitoring Platform
The CrystalSCAN is a bench-top, automated, parallel crystallization monitoring platform, f...


