Discovery
What challenges appear in the discovery phase?
Crystal polymorphism (the precipitation of certain molecules can take different forms, depending on the intermolecular arrangements.1) can result in suboptimal performance. Polymorphs vary in their physico-chemical properties, including solubility and bioavailability, chemical and physical stability, as well as mechanical properties. For this reason, extensive polymorph screening is crucial to identify the optimal structure not only during synthesis but also during use and storage. The complexity of this process and the added lack of knowledge in this field have resulted in the description of crystallization as an art rather than a science.
In silico simulation is used routinely in crystallization to reduce the time and resources used in the discovery phase. However, this methodology also has significant limitations, which can only be addressed by physical testing. Early detection of crystalline forms requires high sensitivity and precision and monitoring multiple parameters simultaneously. This includes close control of factors that impact solubilization processes, such as temperature, and clues about the evolution of the process, such as optical density.
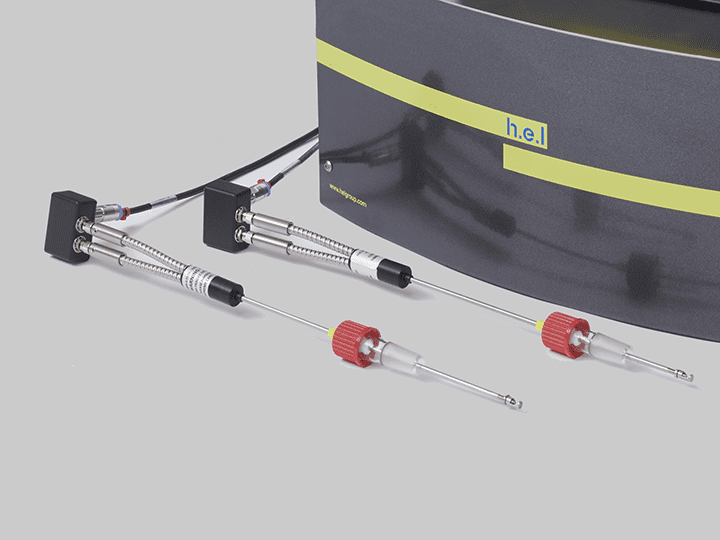
CrystalEYES | Crystallization Monitoring Sensor
Easily Determine Solubility… The need to study crystallization is widespread in the che...
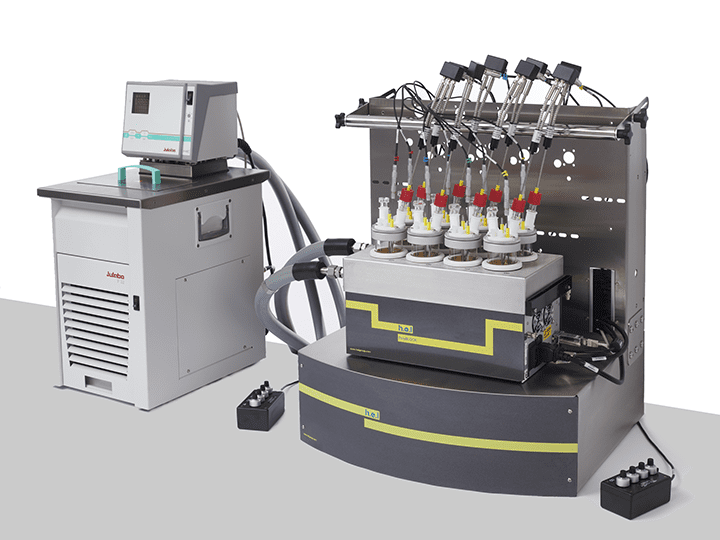
CrystalSCAN | Parallel Crystallization Monitoring Platform
The CrystalSCAN is a bench-top, automated, parallel crystallization monitoring platform, f...
How can reproducibility be increased in the discovery phase?
Crystallization is a highly complex process impacted by numerous factors. Temperature, for instance, significantly affects solubility, impacting both the nucleation and crystal growth processes. Evaporation and cooling, which control the concentration of solute in the mix, are also very heavily influenced by temperature. Minor deviations from the ideal conditions often result in suboptimal outcomes, challenging consistent replication. Automation can significantly enhance reproducibility by minimizing human error and ensuring precise control over experimental conditions2.
Impurities, even in trace amounts, can significantly affect the crystallization process. They may act as nucleation sites, facilitating seed formation and interfering with crystal growth rates, leading to inconsistent results.
Supersaturation is another critical parameter in crystallization, defined as the point where the solute concentration exceeds what is thermodynamically stable. Accurate control and measure of supersaturation are essential for controlling nucleation rates and ensuring consistent crystal formation.
Solutions
CrystalEYES is H.E.L’s crystallization monitoring sensor, which can detect changes in the solution’s turbidity that indicate the precipitation processes. CrystalEYES provides insights into the process, allowing parameter adjustments to optimize conditions to increase the reproducibility and stability of the desired crystal form.
CrystalSCAN is designed for parallel crystallization monitoring and significantly accelerating the screening of parameters in the discovery phase. This is a powerful tool for identifying the optimal nucleation and crystal growth conditions. CrystalSCAN’s ability to determine solubility curves of metastable zone widths makes it a fantastic instrument for understanding the impact of physicochemical variables in the process.

CrystalEYES | Crystallization Monitoring Sensor
Easily Determine Solubility… The need to study crystallization is widespread in the che...

CrystalSCAN | Parallel Crystallization Monitoring Platform
The CrystalSCAN is a bench-top, automated, parallel crystallization monitoring platform, f...


