Discovery
What characteristics are tested in the screening phase?
Screening is an essential component in the process of novel substances of interest. Recyclability, or the capacity to recover the molecule of interest once the reaction has finished, is fundamental. The products of the reactions are detached or desorbed from the catalyst, and it is ready for subsequent processes. This is linked to the second characteristic of interest: stability. Ideally, our chemical of interest would be able to maintain its capabilities to accelerate the chemical reactions at the same level as long as possible during its active life, regardless of the number of times it has been regenerated.
Last but not least, it is fundamental that the catalysts are selective, which means they will overwhelmingly catalyze the reaction, avoiding side reactions and by-products as much as possible. Ultimately, catalysis aims to increase the quality of the chemical reaction, increasing the yield and efficiency. These are both related to the amount of the final product. Whereas the efficiency indicates the amount of reactant necessary to generate a unit of product, the yield also accounts for other variables such as time.
What are the potential challenges in hydrogenation and catalysis processes?
Optimizing chemical reactions, especially those involving hydrogenation and catalysis, requires addressing various physical and chemical factors. Challenges such as maintaining ideal catalyst conditions, preventing hazardous situations, and managing catalyst deactivation and by-product formation must be carefully considered and addressed.
Additionally, extra challenges need to be addressed because of the nature of catalysis. These include the ideal conditions for catalysts. For example, in the case of homogeneous catalysis, the physical conditions need to be such that all the reactants remain in the same physical state, avoiding precipitation processes.
What key features should considered in catalysis and hydrogenation studies?
The discovery phase is a long phase in which different catalysis, substrates, and products must be tested. Parallelization can increase the throughput of this process, enabling researchers to test different substances under the same conditions to identify potential candidates. Alternatively, the same candidate reaction can be tested under different conditions, decreasing the number of tests needed. High-resolution techniques and automation are crucial in characterizing reaction kinetics efficiently, enabling accurate and timely data collection while minimizing human error.
Solutions
H.E.L provides highly customizable and flexible instruments designed for catalyst screening and hydrogenation. Parallel systems offer increased throughput, decreasing the time required to optimize processes. Manual screening can be performed using the CAT range, allowing for up to 7, 18, or 24 individual reactors of different volumes. For higher throughput screening, the DigiCAT 96 allows for up to 96 individual tests using a 96-well Zinsser block, ideal for early catalyst development. The ChemSCAN is a fully automated benchtop catalyst screening platform with four reactors with varied volumes. This allows the ChemSCAN for each reactor to operate independently or in parallel, reducing testing times.
High-pressure is particularly interesting for gaseous reactions (such as hydrogenation) as it favors the solubility of gases in liquid phases. H.E.L’s PolyCAT (4 and 8) are benchtop, automated screening platforms specifically designed for this type of reaction. Additionally, using different reactor volumes (from 16 to 500 ml) can help reduce costs associated with using resources.
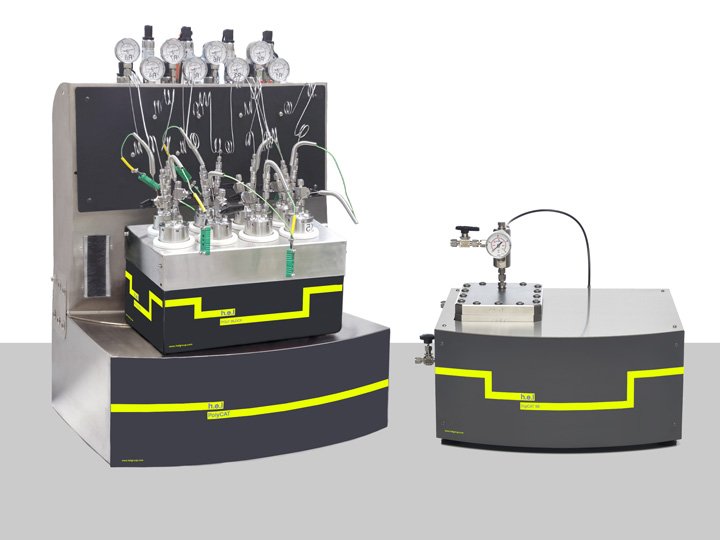
Catalyst Screening Platforms | Manual Systems
Our complete range of multi-sample high throughput catalyst screening vessels has been des...
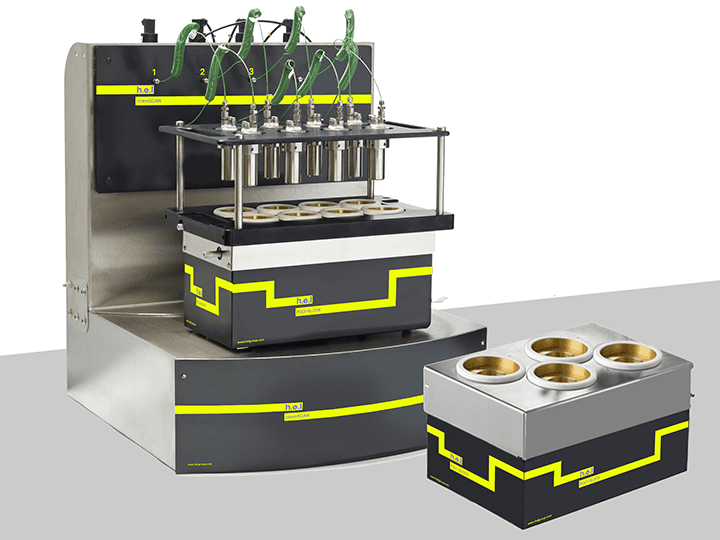
ChemSCAN | Parallel Catalyst Screening and Development Platform
ChemSCAN brings efficiency and flexibility to catalytic research by enabling up to 8 high-...


