Calorimetric analysis of CO2 absorption in an aqueous N-methyldiethanolamine solution using H.E.L.’s Simular
Vilobh Shete1, Dr. Rajendra Kumar2, Mario Toubes-Rodrigo3
1 H.E.L India Private Ltd, 30, Vega, Hiranandani Estate, Thane, Maharashtra, India
2 CSIR-National Chemical Laboratory, Dr Homi Bhabha Road, Pune, Maharashtra, India
3 H.E.L Group, Unit 2 Centro, Boundary Way, Hemel Hempstead, HP2 7SU, UK
Table of Contents
Abstract
The study of the CO2 absorption process in alkanolamines is crucial for enhancing gas sweetening and carbon capture technologies, which are essential in reducing greenhouse emissions. Alkanolamines, such as N-methyl diethanolamine (MDEA), are favored because of their thermodynamic and kinetic properties that improve CO2 capture efficiency. Understanding the calorimetry of these processes aids in optimizing absorption capacity and energy efficiency. Here, we show the thermodynamic behavior of CO2 absorption in a 30% MDEA solution using H.E.L’s Simular isothermal reaction calorimeter operated in power compensation mode. The heat liberated during CO2 absorption in a 30% MDEA solution was determined to be 77.07 kJ mol-1 on CO2 basis. This result demonstrates the capability of Simular to generate calorimetric data for the gas adsorption process. This finding highlights the potential of Simular as a powerful tool for optimizing CO2 absorption processes, contributing to effective and safe carbon capture technologies, and supporting global climate change mitigation efforts.
Introduction
The study of CO2 absorption in alkanolamines is critical for the optimization of industrial processes – e.g., gas sweetening and carbon capture. These processes are essential in the battle against global warming for their capabilities to reduce greenhouse emissions. Alkanolamines, like N-methyl diethanolamine (MDEA) (Figure 1), are frequently used due to their favorable thermodynamic and kinetic properties, which enhance the efficiency of CO2 capture from industrial waste gases1,2. Understanding enthalpy changes using the calorimetry of these absorption processes provides insight into the processes, enabling its optimization in terms of both absorption capacity and energy efficiency.3
Figure 1. N-methyldiethanolamine-molecule
Direct calorimetric measurements of this process provide accurate values on the enthalpy of absorption, reflecting both the heat effects due to the physical dissolution of gas in the solvent and the chemical reaction between CO2 and amine. The key reactions that take place during the process are shown in Figure 2. The heat of absorption of CO2 in aqueous solutions of 27% MDEA (w/v) has been previously investigated, resulting in an average value of 61.4±2.8 kJ/mol 4. High pressure has been shown to increase the solubility of CO2. These studies highlighted the importance of detailed calorimetric analysis in developing efficient and cost-effective carbon capture technologies. The aim of this application note was to use H.E.L’s Simular with a MDEA solution and analyze the thermodynamic behavior of the absorption of CO2 in the aforementioned alkanolamine.
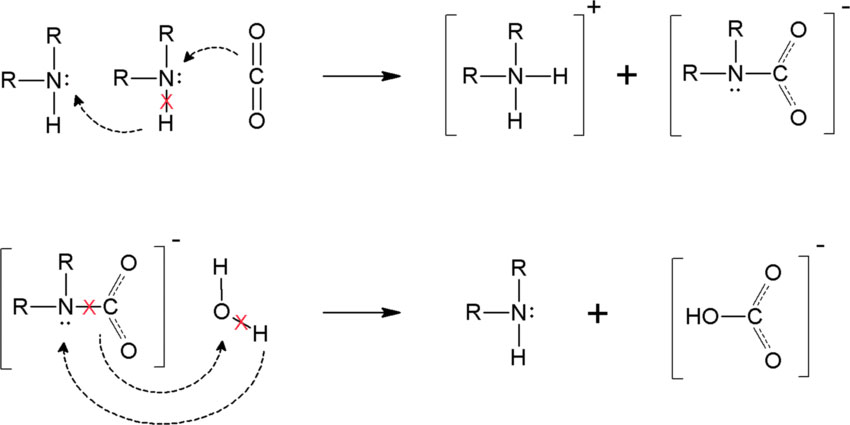
Figure 2. Chemical reaction steps involved in the absorption of CO2 in alkanoamines
Simular
H.E.L’s Simular (Figure 3) is a highly configurable reaction calorimeter designed for precise monitoring and control of reaction parameters. This equipment is designed for the determination of heat generation during a process; calorimetric data can be further used for process optimization and hazard assessment to investigate the thermal properties of a chemical reaction under defined operating conditions. In this application note, a 1-liter stainless steel reactor was used. Brooks Mass Flow Controller (MFC SLA 5850S) was used to feed the CO2 into the reaction mass as well as to maintain the constant reaction pressure inside the reactor. A Druck pressure transducer with a 250 bar pressure rating was used to monitor high-resolution pressure.
The Simular offers the flexibility to operate between traditional Heat Flow or, for quicker results, Power Compensation Control (PCC), which eliminates the need for pre and post-calibration. Simular uses WinISO software with the real-time display and logging of all the process parameters such as reactor temperature, jacket temperature, pressure, power, gas feed flow rate, total gas feed, etc. WinISO integrates operation and data management, providing an intuitive interface to run, monitor, and control the reaction calorimeter experiment. Raw data captured using WinISO can be analyzed separately using the iQ interface to determine the heat of the reaction.

Figure 3 High-Pressure Simular Reaction Calorimeter
Material and methods
A solution of 30% MDEA (w/v) in distilled water was prepared using 98 % MDEA procured from Loba Chemie Pvt Ltd., Mumbai. CO2 gas used was procured from Sandesh Gases Pvt. Ltd., Pune, with 99.99 % purity. 400 ml of 30% MDEA solution was taken into Simular pressure reactor made by SS316L. Simular was operated in power compensation, maintaining the reaction in isothermal conditions at 50 ⁰C temperature. CO2 gas was fed with a regulator and Mass Flow Controller until the pressure reached 6 bar (absolute). Once the desired pressure of 6 bar was reached, CO2 feeding was stopped while maintaining the reaction at constant temperature of 50 ⁰C. The heater power which is the representative data of actual reaction power was monitored and recorded throughout the reaction. Once the heater power reached a stable value, the reaction was considered to be complete.
Results and Discussion
Figure 4 shows the reactor temperature, jacket temperature (Oil in Temp), heater power, and pressure profile of the CO2 absorption in the 30% MDEA solution experiment. In this experiment, the reaction temperature was 50 ⁰C, and the initial reaction pressure was 6 bar. During the first step, the heater did not supply any heat, and the reactor temperature was solely maintained at 50 ⁰C by the circulator. In the second step, the temperature of the circulator was reduced to 40 ⁰C as this was power compensation calorimetry, and a compensation drop of 10 ⁰C was chosen. This temperature difference was compensated by the heater in order to maintain the reaction temperature of 50 ⁰C. Once CO2 is added to the vessel and the chemical reaction starts, energy is released. In order to maintain the reaction temperature at 50 ⁰C and handle the energy released, cooling needs to be provided which was done using the heater, as heater power decreases. Heater power automatically reaches its initial value once the reaction ends and there is no further energy released. Pressure data shows a continuous drop because after reaching the reactor pressure at 6 bar, the CO2 feed was stopped, and due to the CO2 consumption during the reaction, pressure started dropping.
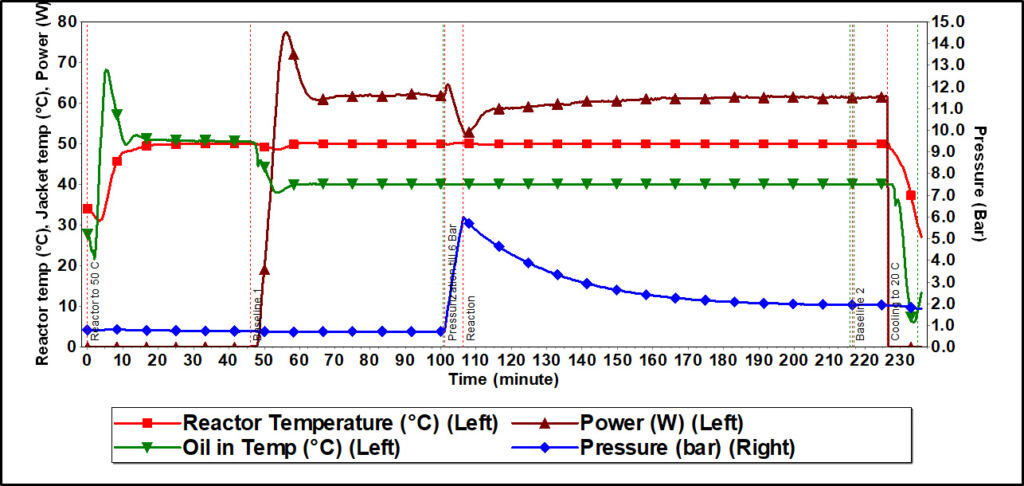
Figure 4. Reactor temperature, jacket temperature, heater power, and CO2 pressure profile from reaction calorimeter experiment of CO2 absorption in a 30% N-methyldiethanolamine solution
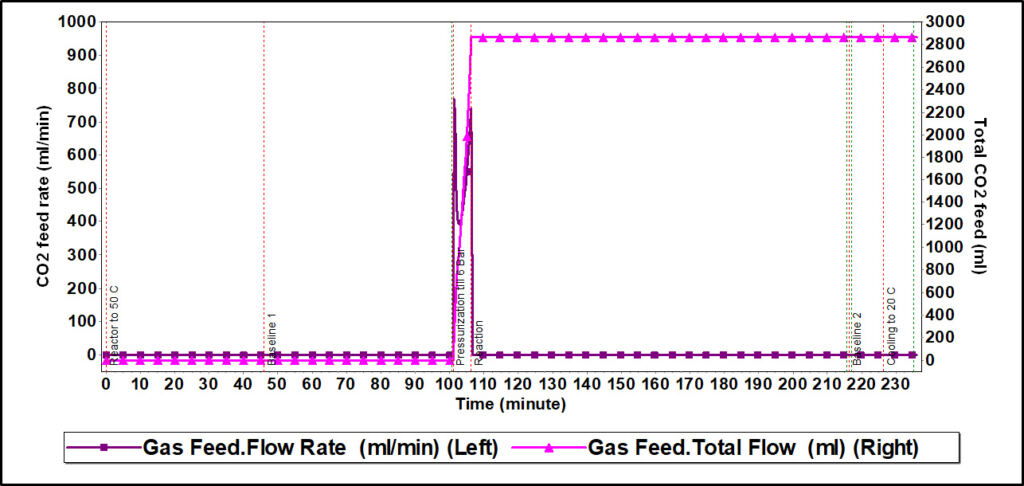
Figure 5. CO2 feed rate and total CO2 feed profiles from reaction calorimeter experiment of CO2 absorption in a 30% N-methyldiethanolamine solution
Figure 4 shows the CO2 feed rate and total CO2 feed profiles from the Simular experiment. In order to reach a reactor pressure of 6 bar, MFC releases a fixed amount of CO2 gas for a certain time only; once 6 bar pressure is reached, MFC automatically stops. That is why after reaching a certain value of total CO2 feed, this profile is completely horizontal with no change with respect to time (pink color).
The heater power profile obtained was integrated using iQ software to estimate the total heat released; it was again converted into molar heat in kJ/mol on a CO2 basis based on the CO2 moles consumption in the process. The value of heat obtained from Simular experiment is the combination of CO2 interaction with MDEA, CO2 interaction with water, heat of mixing, heat loss to the environment, stirrer energy, etc. Hence, the obtained experimental value in the presented work may have some deviation from the theoretical values based on either heat of formation or bond energy methods.
Conclusion
Alkanolamines are highly promising molecules in our battle against climate change due to their capacity to absorb CO2. As this is an exothermic process, hence quantification of its heat of reaction is a must for the process understanding and safe scale-up. The presented experiment and obtained results demonstrate the capability of Simular for the use of this process. A 30 % MDEA solution was used for the presented work. However, a similar result can be obtained for other MDEA concentrations and other industrial alkanolamines. For the presented study, heat of reaction of 77.07 kJ mol-1 was obtained for the CO2 absorption process on 30 % MDEA solution. Moreover, Simular has the capacity to operate in Power Compensation, a method that has been shown to provide faster results with no loss in accuracy or precision5. Simular is, therefore, a very powerful tool for obtaining thermal change data while reducing experiment time and increasing the efficiency of research.
Bibliography
- Rochelle, G. T. Amine scrubbing for CO2 capture. Science 325, 1652–1654 (2009).
- Kohl, A. & Nielsen, R. Gas Purification. Houst. Gulf Publ. Co (1997).
- Dutcher, B., Fan, M. & Russell, A. G. Amine-based CO2 capture technology development from the beginning of 2013 A Review. ACS Appl. Mater. Interfaces 7, 2137–2148 (2015).
- Svensson, H., Hulteberg, C. & Karlsson, H. T. Heat of absorption of CO2 in aqueous solutions of N-methyldiethanolamine and piperazine. Int. J. Greenh. Gas Control 17, 89–98 (2013).
- Toubes-Rodrigo, M. Calorimetry methodologies using Simular reaction calorimeter. H.E.L Group.



