Comparison of different calorimetry methodologies using Simular reaction calorimeter
Dr. Mario Toubes-Rodrigo
H.E.L Group, Unit 2 Centro, Boundary Way, Hemel Hempstead, HP2 7SU
Abstract
Reaction calorimetry is a fundamental tool in the evaluation of chemical processes by measuring heat exchange. In this study, we compare results obtained from Heat Flow (HF) and Power Compensation (PC) methodologies utilizing H.E.L’s Simular. HF is considered the standard methodology in industry, which tracks temperature gradient across the jacket wall in reactors, whereas PC maintains a stable temperature throughout the process by adjusting and measuring differences in electrical power. We assess the performance of both methodologies using acetic anhydride hydrolysis. Results show comparable values for the reaction enthalpy for HF (60.0±2.4 kJ mol-1) and PC (59.1±1.9 kJ mol-1), indicating no significant differences (p-value = 0.27). Despite differing operational principles, both methodologies offer comparable, reliable results. PC, with fewer steps, presents notable advantages, potentially increasing the productivity of the process and reducing costs. This study underscores the viability of PC as a competitive alternative to HF, emphasizing its potential for enhancing process efficiency in chemical industries.
Table of Contents
Introduction
Calorimetry is the science that measures the amount of heat released or absorbed in a process. Reaction calorimetry studies the heat exchange in reactions and is a powerful tool for evaluating chemical processes, including potential risks and hazards. The results from this type of analysis are fundamental to creating safer processes and designing potential mitigation processes. Reaction calorimetry provides information that enables the optimization of chemical reactions (such as dosing profiles or process temperature) and the prediction of the thermal profile during the process, which is fundamental in scale-up methods. Additionally, calorimetry can be used as a tool to optimize existing processes (scale-down) in order to obtain higher productivity.
Heat flow (HF) was the first method to be developed, and nowadays, it is considered the standard methodology in industry. It is based on the use of jacketed reactors equipped with temperature probes. This configuration tracks temperature gradients through the reactor, which allows the quantification of heat flow through the wall. This value will be equivalent to the heat produced or absorbed by the chemical reaction and is represented by the equation:
Q = UA(Tr-Tj)
Where Q is the heat transferred through the wall, U is the heat transfer coefficient, A is the wetted area, Tr is the temperature inside the reactor, and Tj is the jacket temperature.
UA will change throughout the reaction due to changes in volume and the physicochemical characteristics of the mix. To account for this, two calibrations are required, one before the reaction and one afterward. These extra steps result in extended processes. (Fig 1a) .
As an alternative, Power Compensation (PC) also calculates the heat exchanged associated with the chemical reaction; however, in this case, the temperature is maintained at a constant value in the reactor and jacket. To achieve this, the electrical heater submerged in the in the reactors needs to provide energy. The temperature control systems will monitor and adjust the temperature within the vessel, therefore keeping it constant. The amount of power adjusted corresponds directly to the heat exchanged by the sample, and therefore, there is no need for extra calibration steps like in HF (Fig 1b).
The aim of this application note is to demonstrate the reliability of Power Compensation methodology in comparison with Heat Flow, highlighting the benefits of shorter experimental times.
Reaction
The hydrolysis of acetic anhydride has been used extensively for safety studies1. The kinetics of this reaction have been extensively studied using different techniques to measure the extent of the reaction and its rate, and it is accepted that the hydrolysis of acetic anhydride is a first-order reaction with respect to both water and acetic anhydride, especially at low concentration1.
The reaction follows the equation:
(CH3CO)2O + H2O -> 2CH3COOH
The hydrolysis of acetic anhydride is exothermic, releasing heat as acetic anhydride reacts with water, generating acetic acid. The reaction enthalpy varies depending on the conditions (eg. Temperature, pressure), but it is generally reported to be between -57 and -63 kJ mol-1(2 and references there in).
Methodology
Heat Flow calorimetry
Initialization: The temperature of the reactor was set to 40°C with the analogue heater set at a fixed power of 5% for a 30 min step.
Baseline 1: the reactor was set to 40°C with the analogue heater on at a fixed power of 5% for 60 mins.
Calibration 1: the reactor temperature was set at 40°C with the analogue heater on at a fixed power of 15% for 60 mins, in order to obtain the first UA value.
Baseline 2: the heater power was reduced at 5% again for a period of time of 60 mins, aiming to re-establish the values in step no. 2 (Baseline 1).
Feed: the reactor temperature was set at 40°C with the analogue heater on at a fixed power of 5%. The acetic anhydride was fed at 1 g min-1.
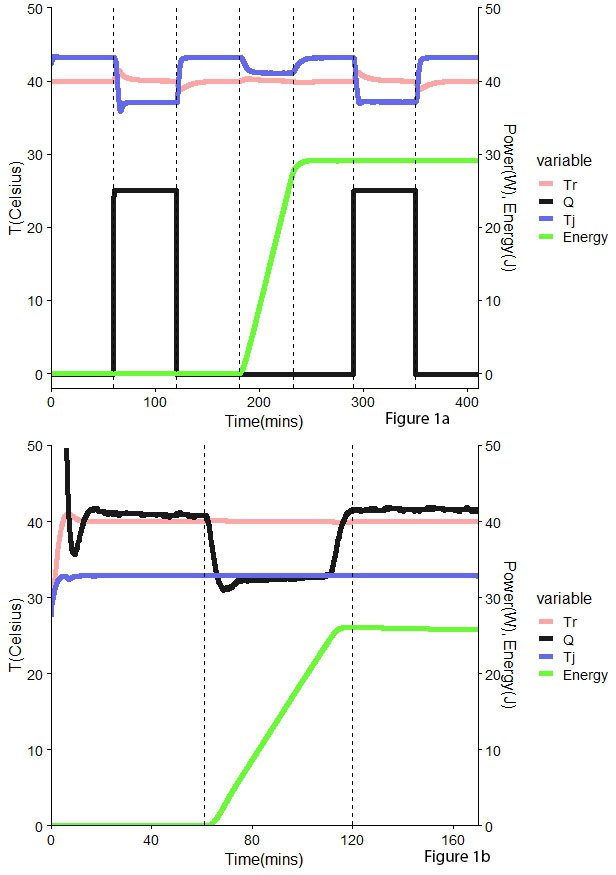
Figure 1. Comparison between Heat Flow (HF) – top – and Power Compensation (PC) – bottom – methodologies. Tr – reactor temperature (°C), Q – heat (W), Tj – jacket temperature (°C), Energy (kJ)
Stir-out: the reactor temperature was set 40°C and the analogue heater on at a fixed power of 5% for a total time of 60 mins to allow the reaction to run to completion.
Baseline 3: the temperature was set at 40°C with the heater at a fixed power of 5% for 60 mins.
Calibration 2: the temperature of the reactor was set at 40°C and the heater on at a fixed power of 15% in order to obtain the second UA value.
Baseline 4: the reactor temperature was set to 40°C, with the analogue heater on at a fixed power of 5%, for 60 mins.
Power Compensation calorimetry
Initialization: the reactor temperature was set to 40°C for 45 mins.
Feed: the heater was supplied with 25V and 5 amp with the aim to maintain the temperature in the reactor at 40°C.
Stir-out: the reactor temperature was set 40°C and the analogue heater on at a fixed power of 5% for a total time of 30 mins to allow the reaction to run to completion.
These two methodologies were repeated in different Simulars (nHF = 23, nPC = 15) with the aim to achieve better repeatability and calculate the accuracy and precision.
Results and Discussion
The reaction enthalpy of the acetic anhydride hydrolysis at 40oC are presented in figure 2. In average, ∆H using Heat Flow was 60.0±2.4 kJ mol-1, whilst the value for Power Compensation was 59.1±1.9 kJ mol-1. The data was normally distributed (Shapiro-Wilk test value 0.95, p-value < 0.05), and a Welch Two Sample t-test were performed, with a p-value = 0.27, revealing that there are no significant differences between both groups.
Reaction enthalpy values in the literature vary between 57 and 632 kJ mol-1. Only one value out of 64 showed a value over the 63 kJ mol-1 threshold (66.11 kJ mol-1). Assuming an average of 60 kJ mol-1 this value still around a 10% of variance.
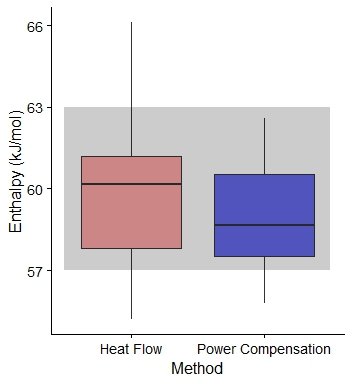
Figure 2. Box plot of the reaction enthalpy value (in kJ mol-1) measured by Simular using Heat Flow (Pink) and Power Compensation (Blue). In grey, reaction enthalpy values in Frede et al.2 and references within. The thick line represents the media value, and the boxplot represents the values between the 2 and 3 quartile.
Assuming accuracy as the mean difference between a value of 60 kJ mol-1 and the measured values, the accuracy for HF is 1.84, and 1.94 for PC, suggesting that there is no real difference this value between both methodologies. Precision-wise, calculated as the mean difference between the average value and the measurements, HF showed a value of 1.84 and the PC, 1.75.
These results showed that both Heat Flow and Power Compensation were perfectly viable methodologies for the characterization of chemical reactions. Whereas, HF measures the heat exchanged between the sample and the reactor jacket, PC accounts for the power adjusted during the process. HF has been the industry standard methodology due to its longevity, however PC has an present an important advantage. A standard measurement using HF requires 9 steps, PC only requires 3. Assuming that each step would be of similar length, this could mean in cutting up process time by two thirds, resulting in significant increases in productivity and reducing costs.
Conclusion
Power Compensation and Heat Flow calorimetry methodologies have significant fundamental differences. However, this Application Notes proves that there are no statistical differences in the reaction enthalpy measured by both methodologies. While Heat Flow calorimetry has long been established as the industry standard in calorimetry, Power Compensation offers the advantage of reducing process times and increasing process productivity.
References
- Garcia, J. M., Bernardino, I. R., Calasans, V. & Giudici, R. Kinetics of the hydrolysis of acetic anhydride using reaction calorimetry: effects of strong acid catalyst and salts. Chem. Eng. Res. Des. 166, 29–39 (2021).
- Frede, T. A. et al. Data Management of Microscale Reaction Calorimeter Using a Modular Open-Source IoT-Platform. Processes 11, 279 (2023).




