Investigating Zinc Oxide-Modified Mordenite as an Effective Catalyst for the Dehydrogenation of Ethanol utilizing the FlowCAT
Russel A. Taylor1*, Samuel J. Raynes1
1 Department of Chemistry, Durham University, South Road, Durham DH1 3LE, UK
In this study, the H.E.L FlowCAT was used for the development and optimization of a process for the production of acetaldehyde via the dehydrogenation of bioethanol. This is the first stage toward a multistep reaction, taking ethanol to higher-value chemicals. This work was carried out at Durham University by Dr. Russell Taylor and Samuel Raynes, to whom all work is accredited. A full copy of the paper is available from the RSC here (1).
Introduction
With in-flow techniques rapidly gaining traction over the past two decades, the scope and viability of flow chemistry processes have increased dramatically (2) As well as enabling unique control over reaction parameters (such as increased temperature control or higher temperature and pressure operating windows), flow chemistry intrinsically aligns itself with Green Chemistry principles such as increased safety and efficiency (3). Dr. Russell Taylor and Samuel Raynes used the H.E.L FlowCAT to develop a process for the production of acetaldehyde from bioethanol in a flow process.
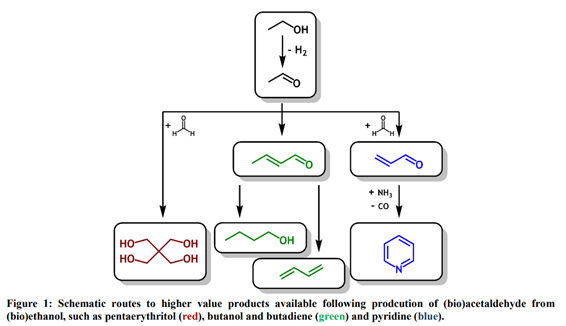
Acetaldehyde is an important precursor for the manufacture of a variety of higher-value compounds, including pyridine derivatives, pentaerythritol, and butanol (4). Acetaldehyde is typically produced industrially via the Wacker process, whereby ethylene is oxidized using a PdCl2/CuCl2 catalyst system, typically giving a 95% acetaldehyde yield at 110 °C and 10 bar. However, the process requires substantial infrastructure investment and predominantly utilizes non-renewable carbon source feedstocks. With the global market for acetaldehyde predicted to grow to around 1.8 billion USD by 2022, a viable method for producing acetaldehyde from renewable feedstocks is desirable (5).
In this study, the H.E.L FlowCAT benchtop flow reactor was used to help develop an in-flow process for the synthesis of acetaldehyde using bioethanol as a feedstock. Bioethanol is produced in large quantities worldwide, with the USA producing bioethanol in excess from the fermentation of corn (6). Direct conversion of bioethanol to acetaldehyde, therefore, represents a more sustainable route to the synthesis of higher-value acetaldehyde derivatives (Figure 1).
The FlowCAT provides an all-in-one flow reactor solution that is optimized for high-pressure catalyst applications. In this study, the FlowCAT provided the necessary flexibility to quickly and easily trial a number of catalysts. Precise control over reaction parameters such as inlet temperature and pressure and reactor temperature enabled optimization of the process for a chosen catalyst, while connection of the FlowCAT’s outlet to a GC-MS-BID enabled real-time analysis of effluent.
Materials and Method
Ethanol can be converted to acetaldehyde via either partial oxidation or direct dehydrogenation. (Equation 1)
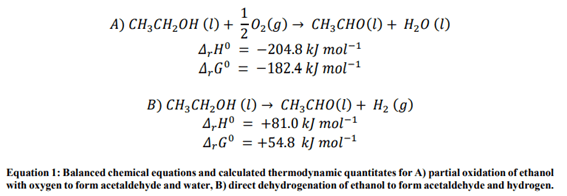
Historically, dehydrogenation of ethanol required such frequent regeneration of the catalyst (typically supported Cu systems) that the partial oxidation route was preferred. Here, researchers used the FlowCAT system to test a number of mordenite-based (MOR) catalysts that had been loaded with various metal oxide species.
Catalysts were prepared by being pressed at 10 tons for 30 seconds in a hydraulic press die equipped with 32 mm pellets. The pressed catalysts were then sieved between 420-250 μm and packed into a stainless steel FlowCAT reactor tube with 6 mm internal diameter. Within the reactor tube, a 1.6 g SiC pre-bed was followed by 0.300 g of the desired catalyst diluted with 1.4 g SiC, then a 2.0 g SiC post-bed.
The FlowCAT provided heat treatment of the catalysts at 150 °C for 1 hour, then at 400 °C for 30 minutes under flowing He or N2 (40 mL/min) before adjusting them to the desired reaction temperature (200-400 °C). This was performed at a controlled ramping rate of 10 °C/min. Once the desired reaction temperature was reached, the system was purged with He or N2 for 30 minutes before ethanol flow was started via an HPLC pump at a rate of 0.171-0.330 mmol/min.
Results and Discussion
On-line analysis was performed in real-time by GC-MS-BID. This enabled the calculation of ethanol conversion, carbon balance, selectivity, yield, and effluent composition (Table S.2).
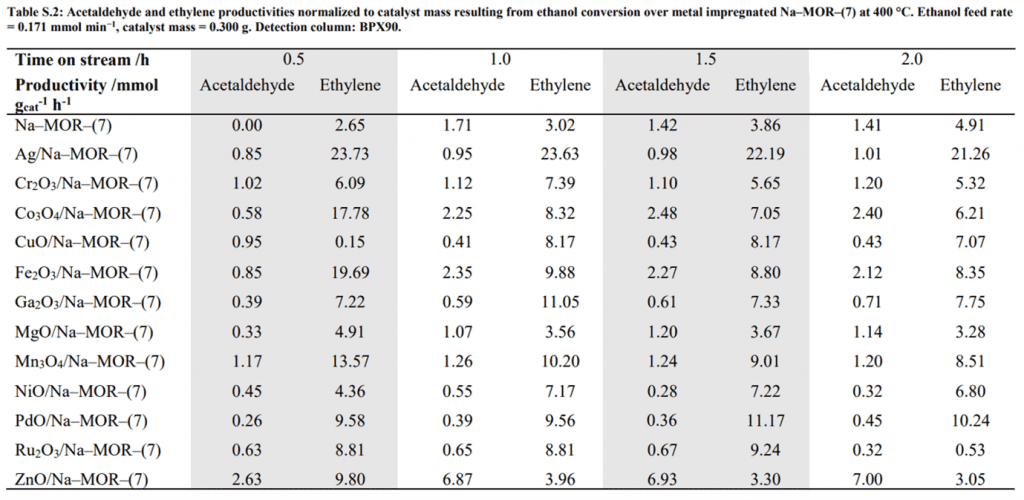
Normalizing results by catalyst mass (A) and by molar metal content (B) both showed ZnO/Na-MOR-(7) to be the most effective catalyst by far in terms of acetaldehyde productivity out of those tested under these reaction conditions. The reaction with this catalyst produced acetaldehyde as the major reaction product, with ethylene as a minor product with low-intensity traces of diethyl ether and 1,3–butadiene.
Further Investigations and System Optimization
With ZnO/Na-MOR-(7) identified as a promising candidate for dehydrogenation of ethanol, the FlowCAT system was used to further investigate optimal parameters for the process.
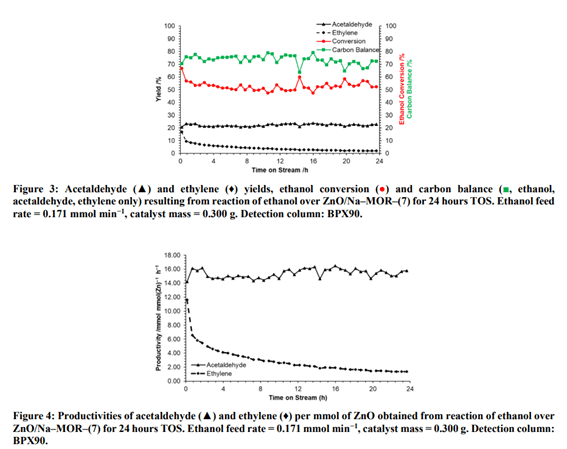
Initially, the reaction of ethanol over ZnO/Na-MOR-(7) was monitored for a period of 24 hours at an ethanol flow rate of 0.171 mmol/min. The FlowCAT and GC-MS-BID systems enabled the yields and productivities of acetaldehyde and ethanol to be closely monitored during this period. This revealed that ethanol conversion and carbon balance remained relatively constant at ~50% and ~70%, respectively throughout the runtime (Figure 3). Additional carbon-containing products were detected (such as CO, CO2, CH4) but not quantified. The missing carbon balance was attributed to these products and visible carbonaceous deposits. It was also observed that while acetaldehyde yield remained steady (~23%) with increasing time on stream, the yield of ethylene was seen to decrease rapidly from ~15% to 3% (Figure 4).
The same system was subsequently used for several more investigations:
- Comparing the effects of different amounts of ZnO loading on acetaldehyde selectivity. Results showed the best acetaldehyde selectivity was achieved at 3.5%-wt.
- Comparing the effects of varying the zeolite counter-cation on acetaldehyde selectivity. It was determined that the Rb+ counter-cation produced superior acetaldehyde selectivity compared with Cs+, K+, and Na+.
Having established ZnO(3.5)/Rb–MOR–(7) as the optimum catalyst for the dehydrogenation of ethanol, the FlowCAT system was used to establish reproducibility and long-term stability. Results showed good reproducibility with around a 14 mmol.g-1cat.h-1 acetaldehyde productivity observed at 50% ethanol conversion at an ethanol flow rate of 0.30 mmol/min. The yield of acetaldehyde remained around 25%, and average carbon balance was maintained above 80% across all replications. Over a 120 hour runtime at an ethanol flow rate of 0.330 mmol/min, a steady acetaldehyde productivity of around 16 mmolg-1h-1 was observed following an initial decrease. No significant deactivation was observed throughout the 120 hour runtime, suggesting long-term catalyst stability.
Conclusion
The FlowCAT system provided a versatile platform with which to assess the performance of several different catalysts for the dehydrogenation of ethanol to acetaldehyde in flow. Once a promising catalyst was identified, the system was used in conjunction with GC-MS-BID for continuous real-time analysis of the process under a range of different parameters, such as catalyst loading and changing the counter-cation. Subsequently, the in-flow process was optimized to the point where it compared favorably with other state-of-the-art systems, providing a promising basis for cost-effective and sustainable industrial acetaldehyde production.
References and Further Reading
1. Zinc Oxide-Modified Mordenite as an Effective Catalyst for the Dehydrogenation of (bio)Ethanol to Acetaldehyde. Samuel John Raynes, Russell Alan Taylor. s.l. : Sustainable Energy Fuels, 2021. DOI: 10.1039/D1SE00091H.
2. The Hitchhiker’s Guide to Flow Chemistry. Plutschack, M.B, Pieber, B., Gilmore, K & Seeberger, P.H. 117, s.l. : Chem. Rev, 2017. 11796-11893.
3. Continuous Manufacturing – The Green Chemistry Promise? Rogers, L. &F. Jensen, K. 21, s.l. : Green Chemistry, 2019. 3481-3498.
4. Acetaldehyde; in Ullmann’s Encyclopedia of Industrial Chemistry. Eckert, M., Fleischmann, G., Jira, R., Bolt, H. M. & Golka, K. 7th Eddition, s.l. : Wiley–VCH Verlag, 2016. 10.1002/14356007.
5. Acetaldehyde Market by Process & Application – Global Forcast 2022. [Online] [Cited: 11 Feb 2021.] https://www.marketsandmarkets.com/Market-Reports/acetaldehyde-market-113225129.html?gclid=CjwKCAjw9vn4BRBaEiwAh0muDFXFtvmo_XxtxX3_cfMiR4GsRR61R3RrC-NFOJJ1zY6WbTRhXZGRwRoC9ZcQAvD_BwE..
6. Annual World Fuel Ethanol Production. U. S. Energy Information Administration. [Online] 2019. [Cited: 11 Feb 2021.] https://ethanolrfa.org/statistics/annual-ethanol-production.





