Several fires linked to E-scooters and e-bikes have recently been featured in the news, and a few years ago, several fires were started on commercial flights related to Samsung Galaxy phones. There is one common denominator between these events, and that is lithium-ion batteries. So, why are lithium-ion batteries prone to failure, and why do we continue to use them despite these risks?
The basic structure of lithium-ion batteries follows the standard electrical cell configuration. They contain a cathode that will serve as the lithium ions’ source and determine the cells’ capacity and voltage; and a cathode that will accumulate and release the lithium ions. These two elements will be submerged in an electrolyte, allowing the movement of ions and a separator to prevent contact between the cathode and anode. Lithium is a very light metal with a very high electrochemical potential, making it incredibly powerful when designing batteries. Still, like a double-edged sword, it is also its main disadvantage. Lithium’s very high electrochemical potential means it is also a very reactive element, and anodes made with this metal also have a low melting point (180oC). Additionally, lithium can crystalize under charge-discharge processes, forming branched structures known as dendrites with high conductivity, which can result in short-circuit events.

Lithium-ion batteries risks
As we have seen, the main risks linked to lithium-ion batteries are battery fires, which are a product of thermal runaways. Thermal runaways are processes in which the heat released from a chemical reaction can accumulate, accelerating the reaction further and resulting in a positive feedback loop. The use of certain electrolytes can exacerbate this situation. In lithium-ion batteries, the electrolytes – normally used are organic carbonates, e.g. propylene carbonate or diethyl carbonate. These chemicals allow lithium salts to dissolve and enable the conductivity to reach levels that permit electrical transport. However, these compounds are susceptible to thermal decomposition, and in this process, flammable gases can accumulate in the battery, which, combined with high temperatures, can result in fire.
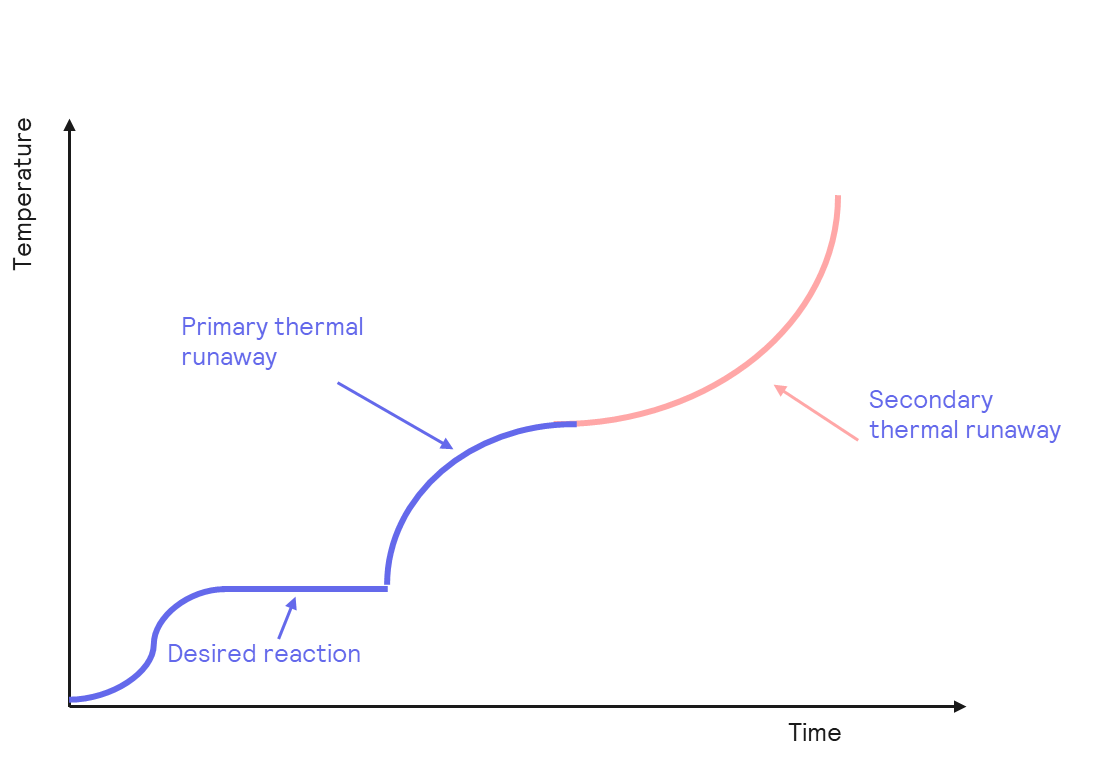
But despite all these downsides, lithium-ion batteries are still used, and there are measures to be put in place that will result in safer batteries. Four main requirements need to be met for a battery to be considered safe, and these are: high conductivity, a stable and controlled solid-electrolyte interface (SEI), the thermal stability of the lithium salt, and the cathode material should not dissolve in the electrolyte. So obviously, the first and the fourth are achieved using lithium salts and a material that is not soluble in the electrolyte, but the second and third one is where there is room for improvement. Using strategies such as temperature-sensitive separators is an option. In this solution, the separator’s material melts when it reaches a certain temperature, increasing the resistance. The extra resistance should be enough to halt the battery, stopping any additional heat production and thus preventing a thermal runaway event.

Using lithium-ion batteries safely
Calorimetry – the study of heat exchange – helps identify the risks and how to address them. To achieve this goal, during the development phase, batteries must be put through potential conditions when they can fail and study the potential effects. Under these scenarios, batteries can behave anomalously, accumulating heat, and being able to record accurately is a great way to prevent accidents from happening. In order for batteries to be sold and distributed, batteries should be rigorously tested and the results recorded. These tests must then be compared to the regulations – e.g. EN 60086-4, IEC 62619, or UL 1642.
When acquiring lithium ion-powered devices, it is fundamental that they are safe to use. Recent reports from December 2022 revealed an increase in fires linked to this kind of battery – 28% on a monthly average – and fire investigators often link these events with poor quality, damaged, or incorrectly charged devices. Market investigations revealed that at least 59 listing online for e-bike and e-scooter chargers fell below minimum understands, including not containing fuses, which would prevent fire in the case of a fault event. So, when using devices powered with lithium-ion batteries, it is fundamental to follow the instructions supplied by the manufacturer and always ensure that they are up to standards and marked with the appropriate seal of approval.
Watch the video
What to learn more?
A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards
Tyne and Wear Fire Service warning after e-scooter fire
‘Alarming’ increase in the number of fires caused by e-scooter and e-bike batteries
Replacement Samsung Galaxy Note 7 phone catches fire on Southwest plane
Fires sparked by e-bike and e-scooters surge by up to 150% in a year as experts warn of dangers of exploding lithium batteries ahead of Christmas sales boom
E-scooter fires caused by lithium batteries
IFSEC Global
Chemistry World