We are an energy-hungry society. Since the advent of the Industrial Revolution in the late 18th century, the need for power has steadily increased, fueling everything from industrial operations to household appliances. Simultaneously, the necessity for efficient energy storage has also grown. Although much has changed since the first batteries were developed by Galvani and Volta, the fundamentals still remain the same. Batteries play a fundamental role in the storage of energy and its release upon demand, ensuring a reliable supply of energy.
However, as we move away from fossil fuels towards a greener and more sustainable society, the demand for better batteries increases: longer-lasting mobile phones and laptops, electric vehicles, scooters, and bikes, and houses powered by renewable energies, just to mention a few. These batteries must also be rechargeable instead of single-use, minimizing the production of unnecessary and very polluting waste, like lead-acid batteries.
So, what makes a battery good?” There is not a single trait that would mean our energy storage is optimal; but rather a combination of parameters that determines its quality. In this blog post, we discuss the 5 top characteristics that the perfect battery needs.
1. Long cycle life
One key parameter to consider when evaluating batteries is their lifespan. Over time, as it undergoes charge/discharge cycles, the battery gradually loses its ability to return to its original capacity. Battery life is determined by the number of times that it can go from fully charged to fully discharged before its total capacity declines below a certain value—normally 80% of the original capacity.
2. High efficiency
Energy efficiency, also known as Coulombic efficiency, measures the ratio of electrons transferred from one electrode of the battery to the other during the charge cycle and those transferred back during discharge. This metric is often highlighted as the single most important parameter to understand the performance of a battery. The energy lost in this process is the result of the internal resistance of the battery, in which energy is lost as heat dissipates. In lithium-ion batteries, this value is very high, generally over 98%. This makes them ideal for applications requiring minimal energy loss and reliable performance over long cycles.
3. High energy density
Energy density refers to the amount of energy a battery can store per unit of mass, making it a crucial parameter in today’s world, where the demand for smaller, more powerful batteries continues to grow. Devices like smartphones and electric vehicles, for example, require more energy over extended periods of time. As such, higher energy density is essential to meet these demands without significantly increasing the size of the battery. However, when higher energy is packed in a cell, the danger of battery failures is higher.
Closely related is power density, which measures the rate of energy output per unit of mass. While energy density focuses on how much energy a battery can store, power density indicates how quickly that energy can be delivered. Both metrics are vital for designing batteries that are both efficient and safe for modern applications.
4. Safety
Batteries are designed to store energy to be released under controlled conditions, but battery failure has become a frequent headline far too often in the last few years. Battery operation can result in a temperature increase. Most of the time, heat is dissipated by the system, but inadequate cooling can result in its accumulation. Certain components of the battery – e.g. electrolyte and cathode – are susceptible to decomposition reactions, when the molecules of which they are made can break down into simpler molecules.
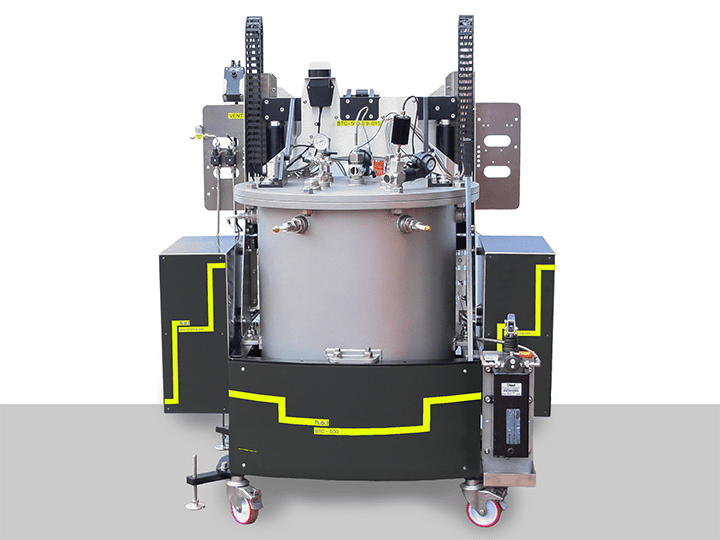 BTC-500 | Large-scale, battery testing, adiabatic calorimeter
BTC-500 | Large-scale, battery testing, adiabatic calorimeter
Decomposition reactions release additional heat, creating a dangerous positive feedback loop called thermal runaway. As the temperature increases, the battery can deform due to the buildup of gases within the cell. These gases are flammable and can react with the oxygen evolved from the decomposition of the cathode, creating a highly volatile mixture susceptible to explosion.
5. Good performance at high and low temperatures
Temperature has a deep impact on the performance of batteries. Often, higher temperatures enhance battery performance, whereas lower temperatures can negatively impact it. However, battery operation outside of the ideal working temperature can severely impact its cycle life. For example, one study showed that Lithium-ion batteries performance decreased by 3.3% when operated at 25°C over 200 cycles. This value doubled when the battery operated at 56°C. Moreover, high temperatures may affect normal battery functioning, increasing the likelihood of thermal runaways, as we discussed in the previous section.

The roadmap to the ideal battery
Developing the “perfect battery” requires a careful balance of several key characteristics. A long cycle life ensures that batteries can be used for extended periods without losing capacity. High efficiency is fundamental, so energy loss is minimized, whilst increasing its performance. Energy density is vital to meet the demands for more powerful devices, albeit compact, being particularly important for devices such as mobile phones and electric vehicles (EVs). However, higher energy density also brings challenges, particularly around safety, due to the higher likelihood of thermal runaways. Additionally, batteries need to be able to operate across a range of temperatures, which can be deleterious for both their performance and longevity. Ultimately, battery innovation needs to focus on optimizing these parameters as we move towards a green future in which we can rely on efficient, safe, and long-lasting energy storage solutions. As research continues, we are likely to see even more advanced batteries that push the boundaries of what is possible in energy storage and usage.