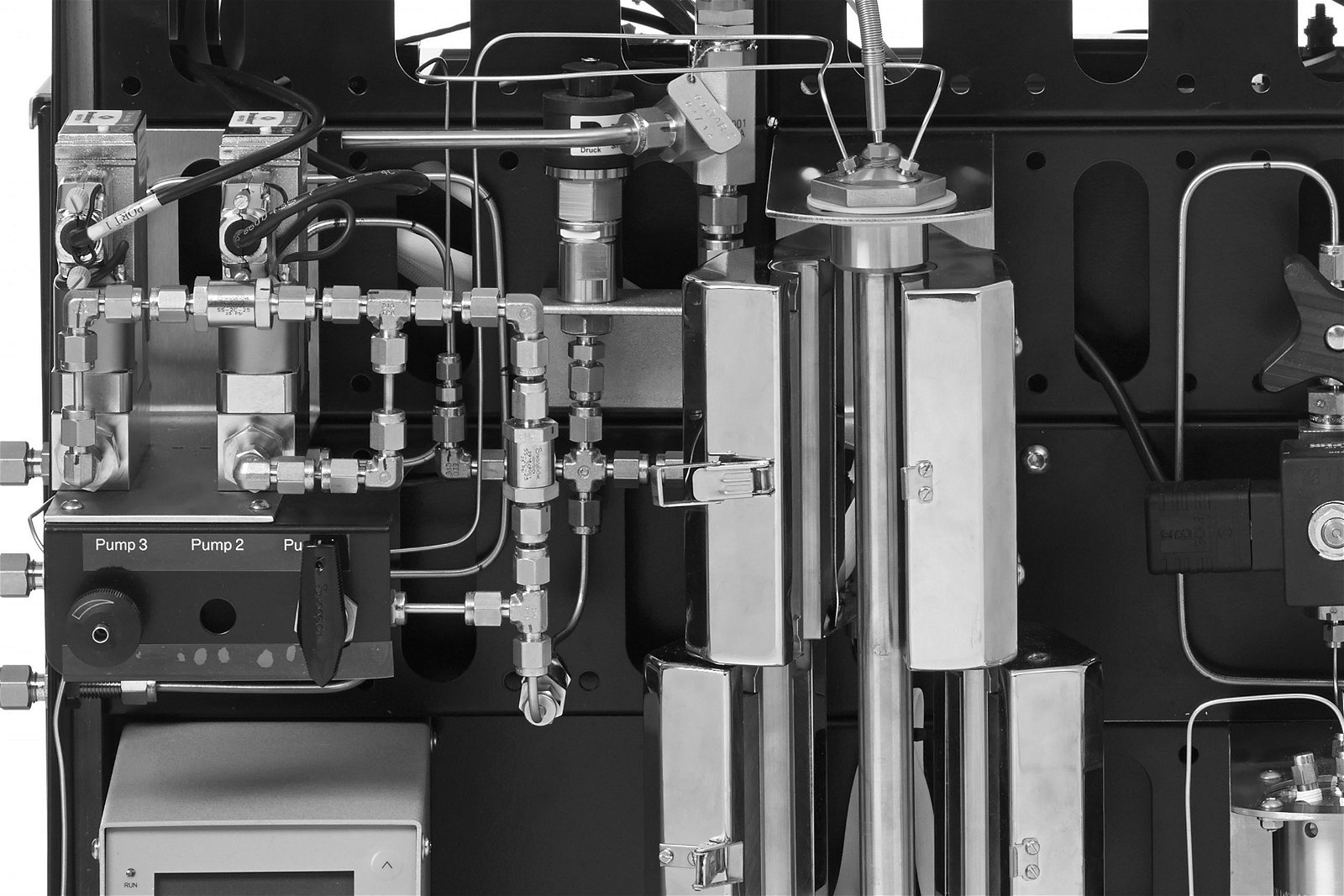
For many, a journey into flow chemistry has just begun; therefore, understanding the key aspects of this process is extremely important.
- Batch Chemistry vs. Flow Chemistry
- Anatomy of Flow Chemistry
- Types of Reactors
- Mixing
- Temperature and Pressure Regulation
- Conclusion
- References
The transition from traditional batch processes to flow chemistry can be quite daunting, especially when you are a first-timer. Our previous blog explains why flow chemistry is more beneficial in the industry than batch reactions. Before using flow processes for your applications, there are so many different aspects to consider. Whether you want to produce large quantities of APIs (active pharmaceutical ingredients) or are looking to test different catalysts, this blog will be your quick guide to flow chemistry.
Batch Chemistry vs. Flow Chemistry
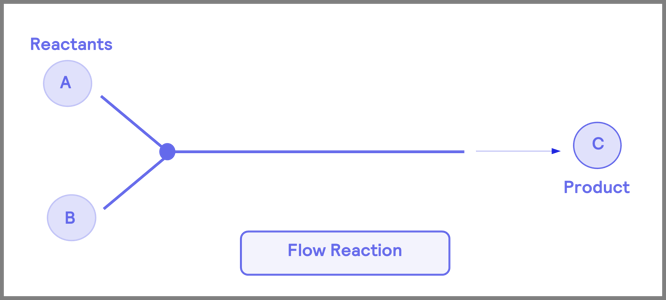
The flow process involves reactants continuously traveling through a reactor vessel to form the desired product (Figure 1). In comparison, multi-step reactions require thorough step-by-step transformations of the initial reactants to achieve the final product. After the isolation step, purification is necessary to remove undesired materials that may disrupt further steps. A significant benefit of flow chemistry is that it allows researchers to bypass the isolation step.
 |
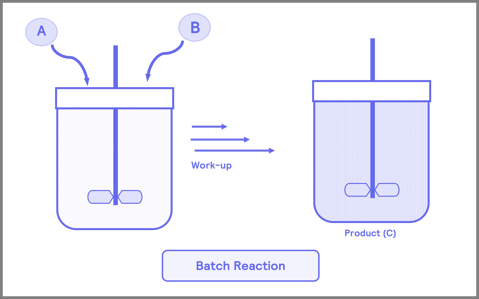 |
Figure 1. Comparison of flow chemistry to traditional multi-step “batch” processes
In flow processes, pumps are used to flow the reaction liquid into the reactor, and this can be achieved using semi-continuous or continuous pumps. Semi-continuous pumps may need a syringe to be refilled; however, continuous pumps do allow for an indefinite flow of solution [1]
Anatomy of Flow Chemistry
A typical flow setup consists of six zones (Figure 2): reagent delivery, mixing, reactor, quenching, pressure regulation, and collection. Purification and further analysis are often also included when the end product is collected.
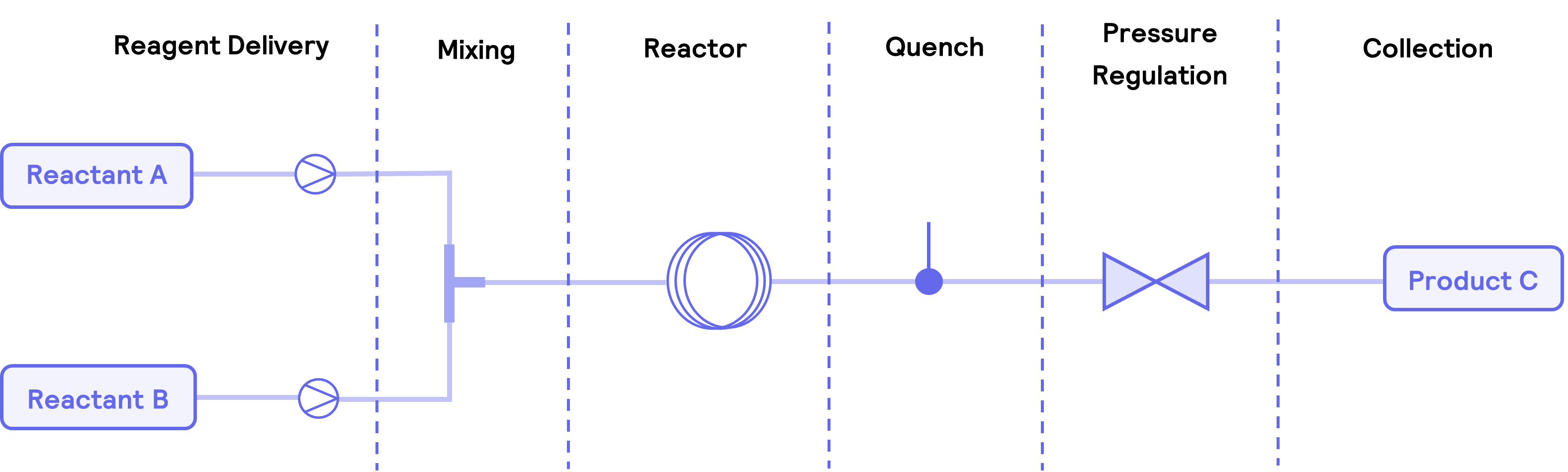 |
Figure 2. A breakdown of the six main components within a basic flow system with a coil reactor. Other stages in the system can include purification and analysis of the final product.
Types of Reactors
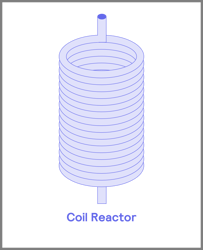
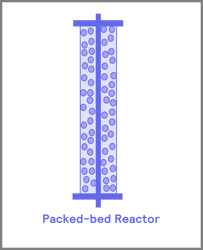
Before you start your flow journey, a number one consideration is the type of reactor that is suitable for your application. These reactor vessels are divided into four main groups: coil, packed bed, chip reactors, and continuous stirred tank reactors [2]. The most used of the three are the packed bed and coil reactors (Figure 3).
 |
 |
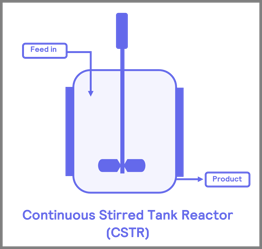 |
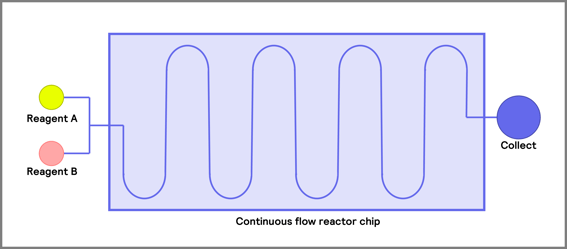 Figure 3. Common reactors used in flow chemical process i) coil ii) packed -bed iii) continuous stirred tank reactor (CSTR) iv) continuous flow reactor chip
Figure 3. Common reactors used in flow chemical process i) coil ii) packed -bed iii) continuous stirred tank reactor (CSTR) iv) continuous flow reactor chip
Coil reactors are mainly used for single-phase chemistry, where the mixing of reagents relies on diffusion rates. Due to the higher cost of chip reactors, coil reactors have become a suitable alternative for synthetic reactions. These reactors tend to be made from fluoropolymers such as PTFE, PFA, and FEP but can also be stainless steel with a range of internal diameters [3].
Packed bed reactors work best for heterogeneous reactions. These reactors are generally tubular and are often stainless steel or Hastelloy to facilitate working at pressure, with a filter frit to ensure particulates do not escape the reactor. Inside the reactor are the catalysts, typically transitional metals, which are coated onto a support material. The support material is then spaced out with some inert packing material, such as glass beads. Fixed bed reactors are highly advantageous for heterogeneous reactions, as the reactants have a greater retention time and thus have a long contact time with the catalyst. Secondly, these reactors achieve a higher effective molarity of catalyst to reagents, decreasing the reaction time.
Mixing
Mixing is a significant component in flow chemistry; flow conditions allow for enhanced mixing and control of reactant residence time. Mixing is influential in the conversion of reactions in flow chemistry. The two main mixing methods are passive and active mixing. Active mixing involves using an agitator to mix the reactants, which can be seen in continuous stirred tank reactors (CSTRs) [3]. Passive mixing, the main form of mixing in our FlowCAT, utilizes pressure that forces fluids to move through the reactor at a constant rate.
Temperature and Pressure Regulation
Temperature and pressure play a significant role in flow chemistry. Along with the flow rate of reactants, temperature and pressure are controlled parameters for the desired reactions to occur. With flow chemistry, heat transfer increases due to the larger surface areas compared with batch methods, allowing for better temperature control.
Pressure control is essential for reaction speeds. In liquid/gas reactions, the reactant gas must efficiently dissolve into the liquid reactant. Increasing pressure allows for increased gas solubility, which will increase the reaction speed. Flow reactions are pressurized using back-pressure regulators, usually found towards the end of the flow system. Back-pressure regulators prevent the liquid and gas flow from the reactor unless the process pressure exceeds a specific threshold.
Conclusion
For many, a journey into flow chemistry has just begun; therefore, understanding the key aspects of this process is extremely important.
Flow chemistry can provide excellent solutions to the chemical industry when utilizing the best and most appropriate technologies to meet demands.
This blog has covered some of these critical points, and we hope it can be the guide you need to start your journey.
References
-
-
- Britton, J., Majumdar, S. and Weiss, G., 2018. Continuous flow biocatalysis. Chemical Society Reviews, 47(15), pp.5891-5918.
- Guidi, M., Seeberger, P. and Gilmore, K., 2020. How to approach flow chemistry. Chemical Society Reviews, 49(24), pp.8910-8932.
- Plutschack, M., Pieber, B., Gilmore, K. and Seeberger, P., 2017. The Hitchhiker’s Guide to Flow Chemistry. Chemical Reviews, 117(18), pp.11796-11893.
- An Overview of Flow Chemistry. Available at: https://www.amt.uk/flow-chemistry (Accessed: 21 October 2021)
-