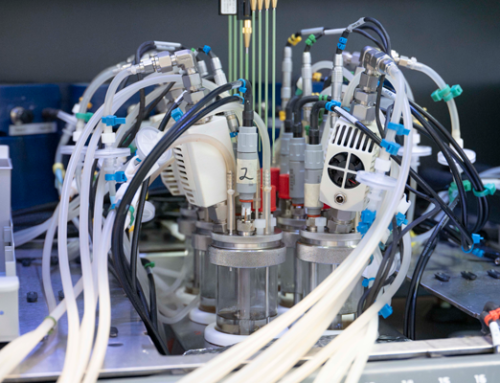
Calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Reaction calorimetry is a potent and helpful tool to evaluate chemical processes and obtain information about non-scalable conditions and potential risks. These risks include increased temperature, pressure, and decomposition of chemical reactions.
Decomposition reactions occur when one reactant, especially if it is unstable, breaks down into two or more products. The progression of a chemical reaction can exacerbate the situation as heat accumulates, and consequentially, the temperature increases over time. As a result, a runway reaction; a dangerous positive feedback loop in which the increased temperature accelerates an unstable exothermic reaction; can be triggered. In turn, further energy released continues to increase the reaction rate. Since 1994 at least 65 major events linked to runaway reactions have been reported in the European Union and in China. Between 1984 to 2019, the count reaches up to 217 instances. In the U.S. Chemical Safety and Hazard Investigation Board has reported at least 167 major accidents involving runaway reactions. In the UK, accidents in the industry linked to runaway reactions are reported to the Health and Safety Executive every two to three weeks.
Understanding chemical reaction risks and safety
A fundamental parameter in the development of safer reactions is the maximum temperature of the synthesis reaction (MTSR), defined as the reaction temperatures plus the adiabatic temperature rise. The adiabatic temperature rise is another fundamental concept in calorimetry. It refers to the change in temperature when there is no gain or loss of heat, so it relates to the changes in temperature due to the chemical reactions occurring in the reactor.
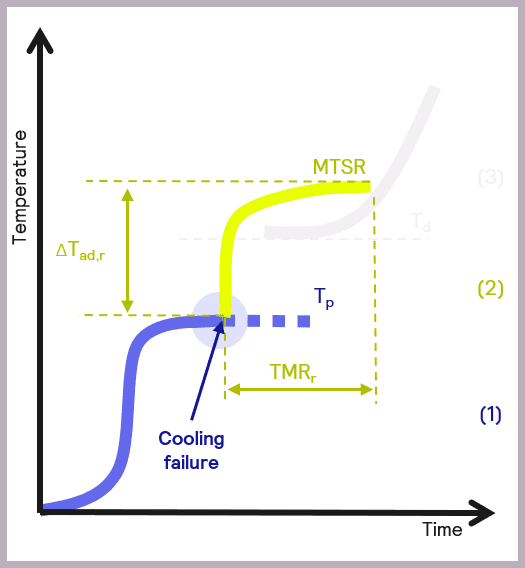
High MTSR values have inherent risks; high temperatures can damage equipment and physically injure people. High temperatures can also result in physical processes of evaporation and increased pressure in reactors, which can lead to explosions. Undesired chemical reactions are another concern: high temperatures can be the perfect conditions for decomposition reactions and produce explosive, flammable, or toxic substances.
However, this is where calorimetry and the use of calorimeters shine. The heat released from chemical reactions scales linearly with the mass of the reactants. So, the ability to simulate the reactions at a smaller scale allows for identifying potential risks. By closely monitoring the reaction, we can see the heat flow and temperatures which helps us understand the kinetics and the behavior of the reactions. Among the things we can learn are the velocity at which the reaction happens and if the reagents accumulate, but also abnormal or unexpected behaviors in the head flow can indicate the presence of secondary reactions. With that information on our hands, we can design control measures to reduce risks.
Towards safer processes
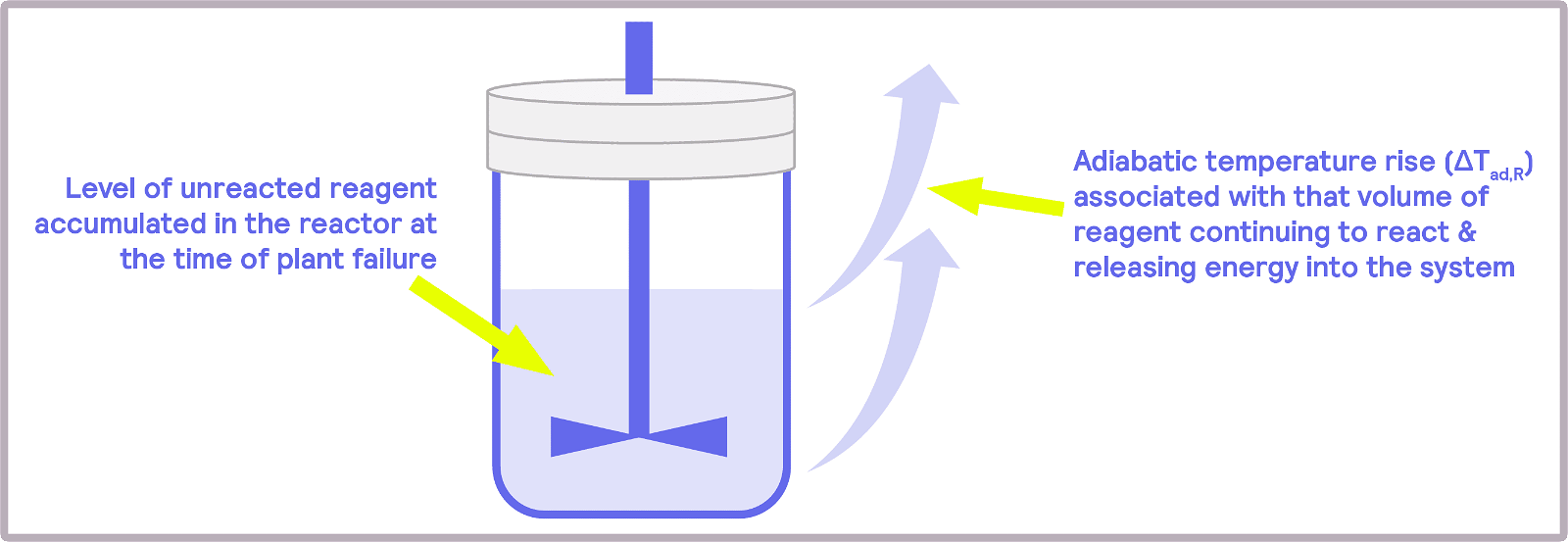
Being able to mimic the process conditions means getting insight into the chemical reaction. With that knowledge, measures can then be put in place. Different scenarios will call for different solutions. An exothermic reaction in which the MTSR reaches values over the decomposition temperature of some components may require cooling. Another issue that reactions may present is that heat can accumulate after the feed process has finished. Batch reactors, in which all reactants are combined, might change to semi-batch reactors, in which one of the reactants can be supplied progressively, avoiding the accumulation of reactants and sudden temperature increases. The ability to adjust the feed rate in semi-batch reactions allows the reaction to progress more slowly and efficiently, preventing a potentially dangerous, sudden accumulation of reactants.
Learning from calorimetry
Sometimes, in a society that continuously pushes towards a continuous increase in production, we forget what we can learn from basic science, and calorimetry is a great example. The careful and thorough study of chemical reactions through calorimetry can teach us important lessons. Understanding if the process is exothermic can help us design mechanisms to keep the temperature increase under safe values. Predicting undesired secondary chemical reactions allows us to avoid sudden over pressurization, which can end up in fatal explosions. Small turning in the rate at which a reactor is fed can result in avoiding the accumulation of reactant, which can lead to the accumulation of energy inside of the reactor and threatening situations. There is so much we can learn from calorimetry, and by applying this knowledge, we can reduce risks, building a safer world.