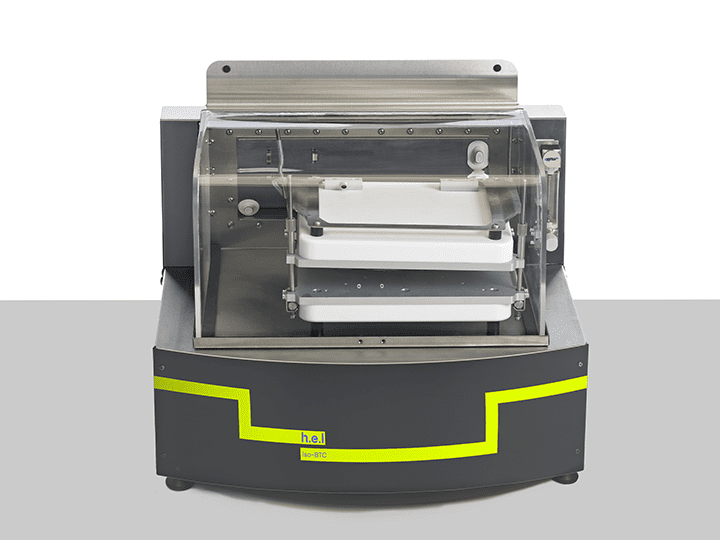
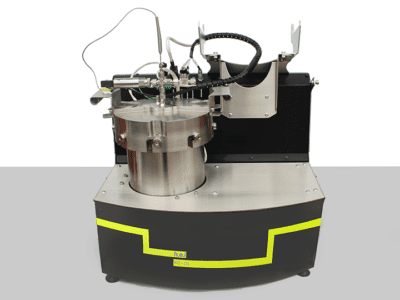
iso-BTC | Bench-top, battery performance testing, isothermal calorimeter
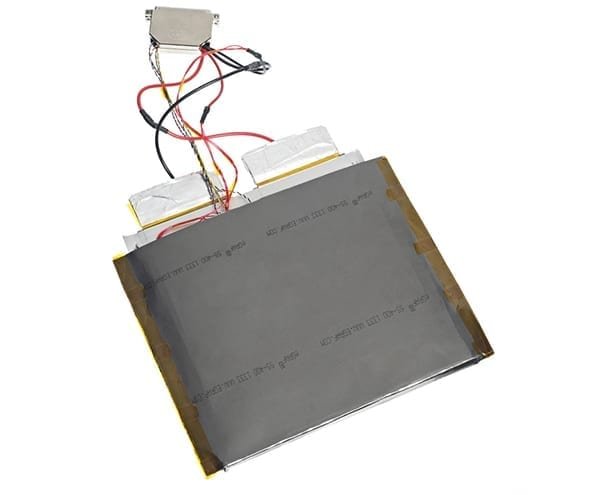
The iso-BTC (Battery Testing Calorimeter) is an isothermal calorimeter designed for the characterization of thermal behavior and electrical performance of battery cells under normal and extended use conditions. It supports a range of adaptors which allows a wide range of cell formats to be analyzed.
The iso-BTC supports the full integration of a charge-discharge unit, allowing for the repeated, automated cycling of battery cells under a range of operating conditions, while concurrently recording both the battery’s electrical performance and the heat evolved. It can also conduct accelerated aging tests, allowing the effect of battery aging on cell performance to be investigated. Battery chemistry, electrode composition, and battery cell type can also be investigated within cell development using the iso-BTC.
Thermal mapping (multipoint temperature measurement) allows the user to identify regions of a battery that generate greater thermal energy to be highlighted. Along with the thermal behavior characterization of the battery cell over a range of temperatures and (dis)charge rates, this information can be used to inform effective and targeted thermal management strategies within the battery module and pack.
Battery Performance and Safety Testing Specifications
Isothermal Calorimeters Brochure
Applications
Performance Testing
Characterize differences in cell performance
- Battery chemistry, electrode composition, type of battery cell, and battery age all influence battery performance. The iso-BTC enables the impact of these factors to be investigated during the development of new cells. The data generated on how battery efficiency, (dis)charging capacity, and heat evolved vary with temperature, and (dis)charging rate can be used to model battery performance and enhance understanding of battery behavior for cell development.
Characterize cell for Quality Control
- Battery attributes, such as battery efficiency and heat evolved, at specified temperatures and C-rates, can be used to characterize the battery performance. These defined characteristics can be used in Quality Control for both cell manufacturers to demonstrate a stated performance, and for battery integrators to check cell performance downstream. The data from an iso-BTC can enable these characteristics to be determined.
Determine thermal management
- Under normal use, heat is absorbed and evolved during the charging and discharging cycles of a battery cell. In addition to this, cells within a module may not exhibit uniform properties upon cycling. The potential imbalance this causes, may trigger a safety hazard and affect battery performance.
- Without careful management, this self-heating can result in overheating and trigger a thermal runaway. The packing and physical arrangement of cells within a module or pack are essential in governing heat transfer. Therefore, it is important to characterize the thermal behavior of the cells, modules, and packs over a range of temperatures and (dis)charge rates, as the data generated can be used to inform effective thermal management.
- The iso-BTC also supports thermal mapping during testing to highlight regions of the battery, which generate greater thermal energy levels. This information can also be utilized in the implementation of targeted thermal management strategies.
Find out more in our guide Solutions in Battery Technology Testing: Hazard screening, safety testing and performance characterization
Application Notes:
On-Demand Webinars:
- Key Benefits of isothermal and adiabatic calorimetry battery testing
- The Use of Isothermal Calorimetry in Battery Performance Testing
Blogs:
- Best Publications on Battery Testing to Read Now – Part 1
- Best Publications on Battery Testing to Read Now – Part 2
Technical Literature
The following is a list of supporting Technical Literature.
How to use an isothermal calorimeter to characterize a gel battery
Publications
The following are a list of some technical publications which highlight the use of the equipment.
Single-crystal structure helps enhance the thermal performance of Ni-rich layered cathode materials for lithium-ion batteries
Xiangbang Kong, Yige Zhang, Jiyang Li, Huiya Yang, Pengpeng Dai, Jing Zeng and Jinbao Zhao
15-Apr-2022
https://www.sciencedirect.com/science/article/abs/pii/S1385894722001462(Subscription or purchase maybe required for full access)
Simplified electrochemical lithium-ion battery model with variable solid-phase diffusion and parameter identification over wide temperature range
Changlong Li, Naxin Cui, Chunyu Wang, Chenghui Zhang
15-Jun-2021
https://www.sciencedirect.com/science/article/abs/pii/S037877532100433X(Subscription or purchase maybe required for full access)
Uncovering LiH Triggered Thermal Runaway Mechanism of a High-Energy LiNi0.5Co0.2Mn0.3O2/Graphite Pouch Cell
Lang Huang, Gaojie Xu, Xiaofan Du, Jiedong Li, Bin Xie, Haisheng Liu, Pengxian Han, Shanmu Dong, Guanglei Cui, Liquan Chen
24-May-2021
https://onlinelibrary.wiley.com/doi/full/10.1002/advs.202100676(Subscription or purchase maybe required for full access)
Reduced-order electrochemical model for lithium-ion battery with domain decomposition and polynomial approximation methods
Changlong Li, Naxin Cui, Chunyu Wang, Chenghui Zhang
15-Apr-2021
https://www.sciencedirect.com/science/article/abs/pii/S0360544220327699(Subscription or purchase maybe required for full access)
Ante-mortem analysis, electrical, thermal, and ageing testing of state-of-the-art cylindrical lithium-ion cells
Hartmut Popp, Ningxin Zhang, Marcus Jahn, Mikel Arrinda, Simon Ritz, Matthias Faber, Dirk Uwe Sauer, Philippe Azais & Iosu Cendoya
16-Jun-2020
https://doi.org/10.1007/s00502-020-00814-9(Subscription or purchase maybe required for full access)
Downloads
The following are a list of available downloads.