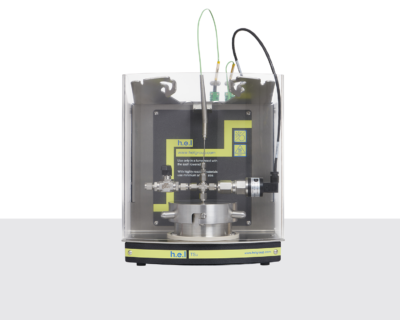
Phi-TEC I | Bench-top, adiabatic calorimeter
The Phi-TEC I is an adiabatic calorimeter that enables the characterization of thermal runaway hazards during process development and scale-up. The Phi-TEC I replicates industrial (large volume) conditions on a lab-scale, enabling thermal runaway hazards to be characterized safely and efficiently.
Via direct sample temperature measurement and by rapidly responding to any thermal changes, the Phi-TEC I accurately tracks exothermic events and maintains adiabatic conditions. This adiabatic screening enables thermal events to be defined with the accurate characterization of the onset temperature (Td) and facilitates the calculation of other key parameters, such as the rate of pressure change, the adiabatic temperature rise (∆Tad,d), and the time to maximum rate (TMRd).
Process Safety and Scale Up Specifications
Industries and Applications
Mining, Explosives manufacture
Special and extremely detailed understanding of explosive reactions needed in order to design devices that…
Research by regulators into hazards of industrial reactions and chemicals to formulate legislation
Widespread testing of hazards using different techniques known and accepted in industry to support both…
Thermal Stability Testing - of APIs, Agrochemicals, propellents
Evaluates decomposition temperatures, onset points, and thermal runaway risks of chemical substances.
Thermal Hazard & Process Safety Solutions
Identify and Mitigate Thermal and Pressure Hazards.
Applications
Rapid reactions
If there is a need to characterize especially rapid decompositions, the Phi-TEC I offers a high data-rate acquisition option, which provides higher resolution data on the rate of pressure and temperature changes. When scaling up a process, accurate knowledge of an exothermic event is vital to ensure the magnitude of the thermal runaway risk is fully understood.
Adiabatic calorimetry
Large scale reactors lose very little of the heat generated in a reaction to the surroundings. This poses a potential hazard when operating at large scale, as that heat will be retained within the reactor. At best this will require plant cooling and at worst may trigger a thermal runaway.
The Phi-TEC I mimics the processes at large scale, while operating at laboratory volumes.
Characterizing the thermal runaway (4)
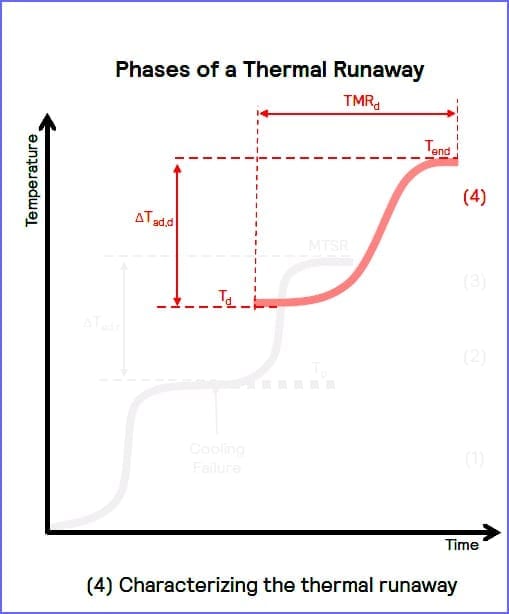 Providing a direct measurement of the sample temperature, coupled with a rapid response to thermal changes, the Phi-TEC I accurately tracks exothermic events and maintains adiabatic conditions.
Providing a direct measurement of the sample temperature, coupled with a rapid response to thermal changes, the Phi-TEC I accurately tracks exothermic events and maintains adiabatic conditions.
Adiabatic screening of a process enables accurate characterization of the onset temperature (Td) and facilitates calculation of the time to maximum rate (TMRd), the adiabatic temperature rise (ΔTad,d) and the rate of pressure change. These key parameters can help describe the magnitude of the thermal runaway hazard.
Technical Literature
The following is a list of supporting Technical Literature.
Creating Process Safety Data Sheets for Safe Scale-Up in Pharma/API Production
Achieving safe conditions for hydrogenation reaction using concentrated raw materials
Publications
The following are a list of some technical publications which highlight the use of the equipment.
Effects of metal ions on thermal hazard of tert-butyl peroxy-3, 5, 5-trimethylhexanoate
Xiang-Hui Shi, Yong Pana, Xin Zhang, Yan-Jun Wang, Li Xia, Jun-Cheng Jiang and Chi-Min Shu
01-Feb-2023
https://www.sciencedirect.com/science/article/abs/pii/S0950423023000037(Subscription or purchase maybe required for full access)
Evaluation of the thermal runaway decomposition of cumene hydroperoxide by adiabatic calorimetry
OJ Reyes-Valdes, VC Moreno & M Sam-Mannan
01-Jan-2023
https://www.researchgate.net/profile/Olga-Reyes-Valdes/publication/298643716_Evaluation_of_the_Thermal_Runaway_Decomposition_of_Cumene_Hydroperoxide_by_Adiabatic_Calorimetry/links/571660a008ae497c1a56feb3/Evaluation-of-the-Thermal-Runaway-Decomposition-of-Cumene-Hydroperoxide-by-Adiabatic-Calorimetry.pdf(Subscription or purchase maybe required for full access)
Thermal hazard assessment of cyclohexane-type liquid crystal monomer from the Grignard cross-coupling reaction by different calorimeters
Wei Wang, Jiancun Gao, Yujing Li, Shengnan Wang, Chenguang Shi, Xiong Dai, Tianmeng Jiang and Guangming Sun
05-May-2022
https://www.sciencedirect.com/science/article/abs/pii/S0040603122000818(Subscription or purchase maybe required for full access)
Effect of reaction type on TMRad, TD24 and other data obtained by adiabatic calorimetry
Xiao-juan Wu, Yi Zhu, Li-ping Chen, Wang-hua Chen & Zi-chao Guo
26-Jun-2021
https://link.springer.com/article/10.1007/s10973-021-10787-2(Subscription or purchase maybe required for full access)
Research on Thermal Decomposition Kinetics and Thermal Safety for a New Epoxiconazole Crystal
Zhen-Yun Wei, Ji-Shuang Tan, Xiao-Hua Ma, Rong Kong, Xuan Liu, Chun-Sheng Cheng & San-Xi Li
16-Feb-2021
https://pubs.acs.org/doi/full/10.1021/acsomega.0c05988(Subscription or purchase maybe required for full access)
Determination of SADT and TMRad of 3-bromo-1-(3,5-dichloropyridin-2-yl)-4,5-dihydro-1H-pyrazole-5-carboxylic acid: Applying thermal decomposition kinetics
Yun-Bo Cong, Zhen-Yun Wei, Xiao-Hua Ma, Zi-Liang Li, Quan-Guo Li, Xu Ming & Chun-Sheng Cheng
31-Jan-2021
https://www.sciencedirect.com/science/article/pii/S2211715621000175(Subscription or purchase maybe required for full access)
Continuous Safety Improvements to Avoid Runaway Reactions: The Case of a Chloro-Thiadiazole Intermediate Synthesis toward Timolol
Alessandro Agosti, Silvia Panzeri, Federico Gassa, Massimo Magnani, Giulia Forni, Marco Quaroni, Lazzaro Feliciani and Giorgio Bertolini
19-May-2020
https://doi.org/10.1021/acs.oprd.0c00048(Subscription or purchase maybe required for full access)
A kinetic-based approach in accelerating rate calorimetry with the varying thermal inertia consideration
Jiong Ding, Liming Yu, Xiaona Wang, Qiyue Xu, Suijun Yang, Shuliang Ye & Juncheng Jiang
25-Nov-2019
https://link.springer.com/article/10.1007/s10973-019-09081-z(Subscription or purchase maybe required for full access)
Thermal hazard assessment of tris(2-methyl butyl) phosphate: A comparison with tri-n-butyl phosphate and tri-n-amyl phosphate
Subramee Sarkar, K. Chandran, M. Lavanya, N. Ramanathan, P.C. Clinsha, A. Suresh & N. Sivaraman
01-Jan-2019
https://www.sciencedirect.com/science/article/abs/pii/S0040603118304441(Subscription or purchase maybe required for full access)
An Alternative Scalable Process for the Synthesis of 4,6-Dichloropyrimidine-5-carbonitrile
Bin Zhang, Huixin Yan, Chongfeng Ge, Bo Liu and Zhenping Wu
01-Nov-2018
https://doi.org/10.1021/acs.oprd.8b00154(Subscription or purchase maybe required for full access)
Thermal decomposition behaviour of irradiated tri n-butyl phosphate and mixture of di and mono n-butyl phosphate-nitric acid systems
K. Chandran, B. Sreenivasulu, N. Ramanathan, P.C. Clinsha, A. Suresh, N. Sivaraman
10-Nov-2017
https://www.sciencedirect.com/science/article/abs/pii/S0040603117302393(Subscription or purchase maybe required for full access)
Studies on thermal decomposition behaviour of N,N-dialkyl octanamides
K. Chandran, C.V.S.Brahmmananda Rao, N. Ramanathan, N. Sivaraman & S. Anthonysamy
10-Jun-2016
https://www.sciencedirect.com/science/article/abs/pii/S0040603116300673(Subscription or purchase maybe required for full access)
Effect of gamma irradiation on thermal decomposition of tri-iso-amyl phosphate–nitric acid biphasic systems
B. Sreenivasulu, K. Chandran, P. C. Clinsha, A. Suresh, N. Sivaraman & S. Anthonysamy
17-Mar-2016
https://link.springer.com/article/10.1007/s10973-016-5375-0(Subscription or purchase maybe required for full access)
Experimental sensitivity analysis of the runaway severity of Dicumyl peroxide decomposition using adiabatic calorimetry
Olga J. Reyes Valdesa, Valeria Casson Moreno, Simon P. Waldram, Luc N. Véchot, M. Sam Mannan
10-Oct-2015
https://doi.org/10.1016/j.tca.2015.07.016(Subscription or purchase maybe required for full access)
Mathematical methods for application of experimental adiabatic data – An update and extension
Arcady A. Kossoy, Jasbir Singh, Elena Yu Koludarova
24-Nov-2014
https://www.sciencedirect.com/science/article/abs/pii/S0950423014002071(Subscription or purchase maybe required for full access)
Thermal decomposition behaviour of diglycolamide – Nitric acid system
K. Chandran, K.A. Venkatesan, S. Anthonysamy & T.G. Srinivasan
10-May-2014
https://www.sciencedirect.com/science/article/abs/pii/S0040603114000938(Subscription or purchase maybe required for full access)
Novel validation on pressure as a determination of onset point for exothermic decomposition of DTBP
Yih-Shing Duh, Wen-Fang Wang & Chen-Shan Kao
26-Feb-2014
https://link.springer.com/article/10.1007/s10973-014-3690-x(Subscription or purchase maybe required for full access)
Thermal decomposition characteristics of octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide-tri n-butyl phosphate–nitric acid systems
K. A. Venkatesan, K. Chandran, N. Ramanathan, S. Anthonysamy, V. Ganesan & T. G. Srinivasan
20-Nov-2013
https://link.springer.com/article/10.1007/s10973-013-3504-6(Subscription or purchase maybe required for full access)
Thermodynamics and kinetics of thermal decomposition of dibutylalkyl and dipentylalkyl phosphonate-nitric acid systems
K. Chandran, C.V.S. Brahmmananda Rao, S. Anthonysamy, V. Ganesan & T.G. Srinivasan
10-Oct-2013
https://www.sciencedirect.com/science/article/abs/pii/S0040603113003857(Subscription or purchase maybe required for full access)
Calorimetric studies on thermal decomposition of tri isoamyl phosphate–nitric acid systems
Tarun Kumar Sahoo, K. Chandran, P. Muralidaran, V. Ganesan & T.G. Srinivasan
20-Apr-2012
https://www.sciencedirect.com/science/article/abs/pii/S004060311200041X(Subscription or purchase maybe required for full access)
Downloads
The following are a list of available downloads.