Picture this. You are placidly sleeping in your home in a quiet town, and you are suddenly woken up by a distant explosion. Minutes later, the sky darkens, and a toxic cloud comes your way. Sadly, there have been several instances of such events. For example, in Seveso (Italy), in 1976, or in Bhopal (India), in 1984.
Both events were linked to a recurring guest in our blog posts, thermal runaways. A thermal runaway occurs when exothermic reactions release energy to the surroundings. If the system is not able to dissipate this heat, the system will reach a high temperature. As the temperature increases, so does the rate at which the chemical reactions occur, resulting in a positive feedback loop, with potentially catastrophic consequences, including explosions and the release of hazardous materials. However, the situation is not as dire as it sounds, and in the last four decades, there has been a continuous effort to reduce risks linked to chemical reactions. Such events resulted in the dawn of Process Safety Engineering.
In this blog post, we will discuss one of the tools used in Process Safety Management: the Stoessel’s Criticality Index.
The 4 players…
Francis Stoessel created a framework1 to determine the inherent risks of chemical processes and the mitigation strategies required to prevent accidents. To achieve this goal, he defined four critical temperatures and the relationship between them.
Process Temperature – Tp: the temperature at which the process runs before the cooling failure scenario.
Maximum Temperature of Synthesis Reaction—MTSR: The MTSR represents the maximum temperature the reactor would reach once all the accumulated reactant is consumed. It is calculated by adding the operating temperature of the process to the temperature increase caused by the reaction as it progresses. This value, therefore, depends on the process design (e.g., batch, semi-batch, or continuous)
TD24 – this is the initial temperature for an adiabatic process (without heat loss) where the Time to Maximum Rate is 24 hours. It represent the temperature at which a runaway reaction reaches its maximum rate within 24 hours.
Maximum temperature for technical reasons – MTT : depending on the system, it can be the boiling point (in the case of open systems), or the temperature at which the pressure is the maximum permissible
… and 5 potential cases
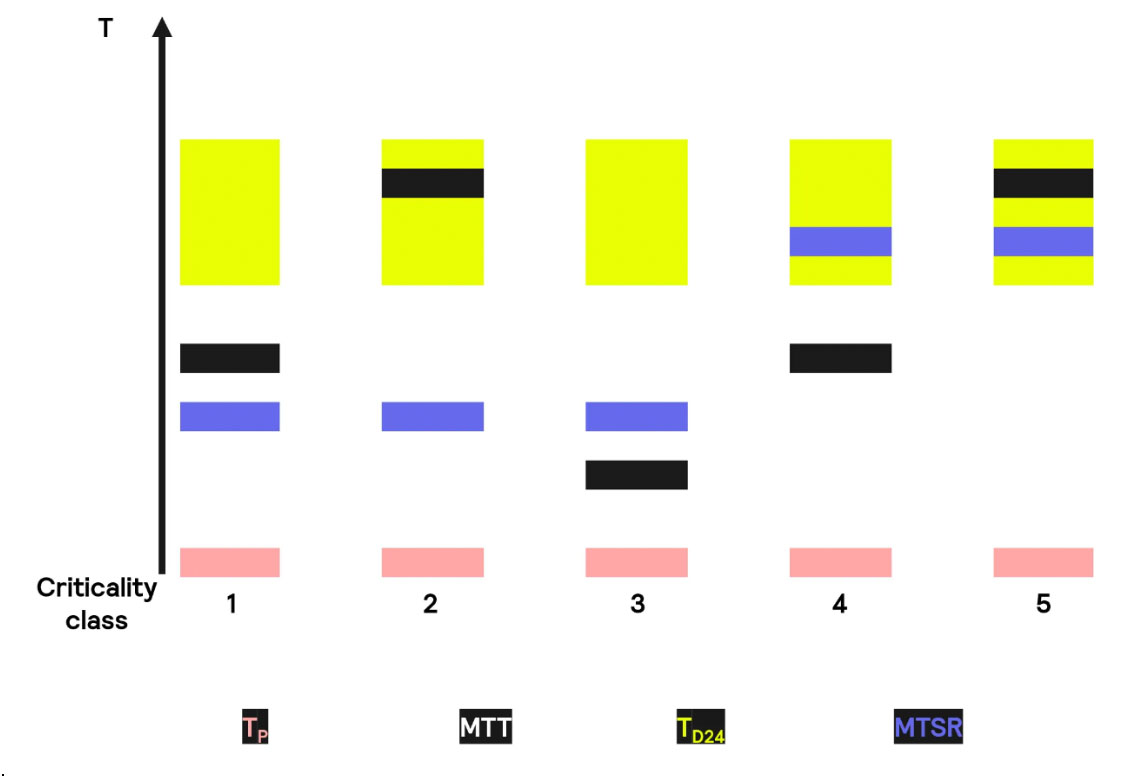
With these 4 temperatures in mind, and depending on how their values compare to each other, 5 different scenarios arise. Let us look into them.
Case 1—Safe Reaction: Both MTT and MTSR are under the TD24, so this situation is deemed safe. However, there are still potential hazards; for example, the total solvent available is vaporized if the heat dissipation is not sufficient.
Case 2 – Limited Risk of Thermal Runaway: the MTSR is less than the TD24. However, in this case, the MTT is lower than the TD24, acting as a barrier that prevents the reaction from reaching the TD24
Case 3 – Risk of Thermal Runaway due to Cooling Failure: The MTSR is higher than the TD24. This means that the MTT does not behave like a safety barrier, and cooling requires close monitorization
Case 4—Thermal Runaway Possible Under Normal Conditions: The MTSR is higher than the TD24. However, the MTT is lower than both values, acting as a first layer of protection.
Case 5 – Immediate Risk of Thermal Runaway : in this case the MTSR is also higher than TD24. Contrary to the previous case, MTT is higher than TD24. This makes this process highly dangerous, and if possible, it should be avoided, since it may be that not even cooling would be enough.
Looking for a safer future
Stoessel’s Criticality Index has been around for the last 15 years, and since then, it has become a vital tool for designing safer chemical engineering processes by estimating the risks of thermal runaways. As industries aim to increase their production rates and efficiency, the potential for hazardous situations also grows. Stoessel’s index provides a systematic approach to assess the risk of potentially dangerous situations. Reactions are categorized based on heat generation versus critical barriers, including physical stops (such as the boiling temperature) and cooling capacities. By doing so, it offers engineers clear guidelines on whether a reaction can proceed safely (case 1), requires safety measures (cases 2 and 3), or should be redesigned entirely (cases 4 and 5). This structured risk assessment not only enhances the safety within the plant but also protects surrounding communities and environments, helping to prevent accidents like in Seveso (1976), Bhopal (1984), and Toulouse (2001).
Additionally, Stoessel’s index encourages a proactive approach to safety, making it possible to design processes with inherent stability instead of relying exclusively on external control or emergency interventions. In a society moving towards sustainability, this approach fits perfectly, reducing the likelihood of accidents and minimizing the need for resource-intensive safety measures. By incorporating safety directly into the design of the chemical process, Stoessel’s index contributes to a future with efficient and secure industrial operation in a more sustainable and resilient chemical industry.