In this section, you will find a library of specific case studies/application notes demonstrating the extensive functionality of how scientists are using H.E.L equipment in all the fields of work, including research, scale-up, and production. These case studies show real results and demonstrate our equipment’s performance in applications for biotechnology, thermal hazards, catalysis, calorimetry, flow chemistry, parallel processing, crystallization, and particle studies. These application notes and case studies are subject to copyright. To use any part of this work, please contact us.
Calorimetric analysis of CO2 absorption in an aqueous N-methyldiethanolamine solution using H.E.L.’s Simular
The study of the CO2 absorption process in alkanolamines is crucial for enhancing gas sweetening and carbon capture technologies, which are essential in reducing greenhouse emissions. Alkanolamines, such as N-methyl diethanolamine (MDEA), are favored because of their thermodynamic and kinetic properties that improve CO2 capture efficiency. Understanding the calorimetry of these processes aids in optimizing absorption capacity and energy efficiency. Here, we show the thermodynamic behavior of CO2 absorption in a 30% MDEA solution using H.E.L’s Simular isothermal reaction calorimeter operated in power compensation mode. This finding highlights the potential of Simular as a powerful tool for optimizing CO2 absorption processes, contributing to effective and safe carbon capture technologies, and supporting global climate change mitigation efforts.
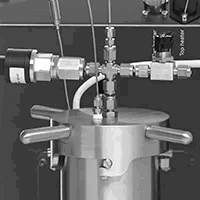
Achieving safe conditions for hydrogenation reaction using concentrated raw materials
Hydrogenation involves the addition of hydrogen gases into molecules, transforming bonds (e.g. C=C to C-C). Hydrogen is highly flammable and explosive, and as such, hydrogenation reactions and their products need to be tested in advance. In this application note, we present the thermal behavior of an alkane and alkene involved in the hydrogenation reaction measured in a Phi-TEC I. Results showed that the alkene was susceptible to decomposition reactions when it reached a temperature 238.8 oC, where the alkane did not display such behavior. Phi-TEC I is an adiabatic calorimeter that has shown its capabilities to detect thermal events in reactants involved in risky hydrogenation reactions.
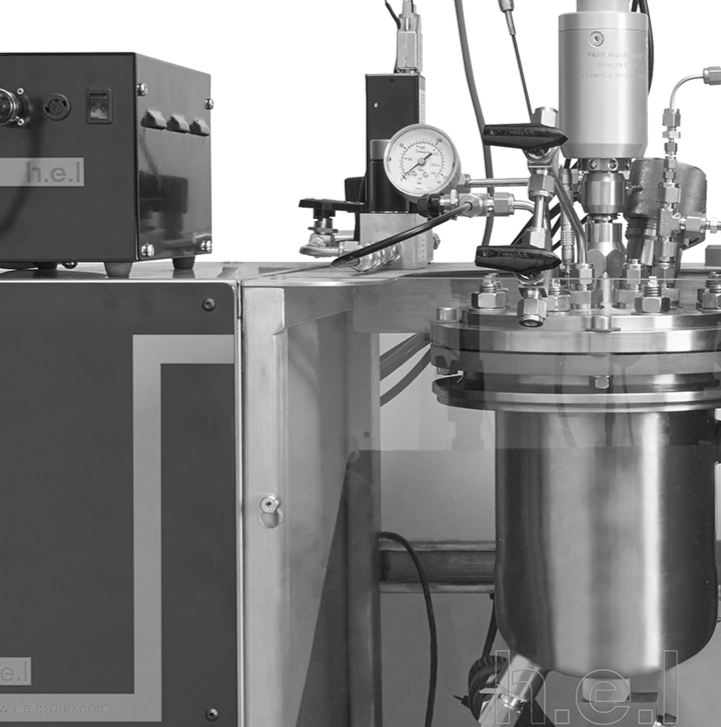
Comparison of different calorimetry methodologies using Simular reaction calorimeter
Reaction calorimetry is a fundamental tool in the evaluation of chemical processes by measuring heat exchange. In this study, we compare results obtained from Heat Flow (HF) and Power Compensation (PC) methodologies utilizing H.E.L’s Simular. HF is considered the standard methodology in industry, which tracks temperature gradient across the jacket wall in reactors, whereas PC maintains a stable temperature throughout the process by adjusting and measuring differences in electrical power. We assess the performance of both methodologies using acetic anhydride hydrolysis.

Calorimetry: Heat flow versus power compensations methods
In this note, we compare the fundamentals and operation of two calorimetry methodologies that the Simular performs: heat flow and power compensation. The mathematical calculations to calculate the energy produced or absorbed by the chemical reaction are presented. The last section directly compares both methods in terms of measurements, temperature control, accuracy, and time.

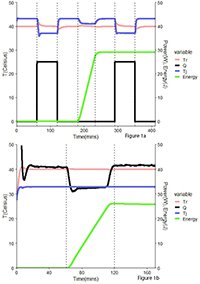
Using the Phi-TEC I in Thermally Unstable Environments
In this study, environmental thermal instability was deliberately introduced while using the Phi-TEC I adiabatic calorimeter for exothermic reaction detection.


