In this section, you will find a library of specific case studies/application notes demonstrating the extensive functionality of how scientists are using H.E.L equipment in all the fields of work, including research, scale-up, and production. These case studies show real results and demonstrate our equipment’s performance in applications for biotechnology, thermal hazards, catalysis, calorimetry, flow chemistry, parallel processing, crystallization, and particle studies. These application notes and case studies are subject to copyright. To use any part of this work, please contact us.

Application of the INFOGEST Protocol Using the BioXplorer 100 for Automated Digestion Simulation
Digestion involves the breakdown of food into smaller compounds to facilitate nutrient release and absorption along the digestive tract. Studies have highlighted the significant impact of digestion on health, which led to the development of a variety of in vitro digestion protocols that produced non-comparable and often inconsistent results.Studies have highlighted the significant impact of digestion on health, which led to the development of a variety of in vitro digestion protocols that produced non-comparable and often inconsistent results

Integration of mass spectrometry to characterize the gas evolution of batteries in thermal runaways using adiabatic calorimetry.
Lithium-ion batteries are an essential energy storage technology, but their increasing energy density raises significant safety concerns. Reports of thermal runaway incidents highlight the urgent need for improved methods to study and mitigate these hazardous events. A key challenge in battery safety research is understanding the gas evolution during thermal runaway, as conventional techniques such as gas chromatography operate under conditions that do not fully replicate high-pressure, high-temperature environments. Here, we present a novel integration of adiabatic calorimetry (BTC-500) with high-pressure mass spectrometry to characterize gas evolution during lithium-ion battery thermal runaway. This approach enables real-time, high-frequency gas composition analysis under extreme conditions, overcoming limitations of conventional gas analysis techniques. Compared to standard gas chromatography, which requires sample transfer and offline analysis, in-situ mass spectrometry provides a direct and dynamic measurement of evolving gases.

Identifying the best rhodium-modified catalyst and the best reaction conditions with ChemSCAN for the synthesis of aroma compounds
In the perfumery industry, there is a constant need to provide compounds imparting novel organoleptic notes. In this study, a ChemSCAN is used to investigate the hydroformylation of the substrate 4,4-dimethyl-1-vinylcyclohexyl acetate as a precursor for the desired 3-(cyclohex-1-en-1-yl)propanal derivative. Fast parallel trials allowed for the identification of the most active and selective modified rhodium catalyst. Subsequent concurrent experiments enabled the evaluation of different catalyst loadings and minimized the amount of solvent employed.
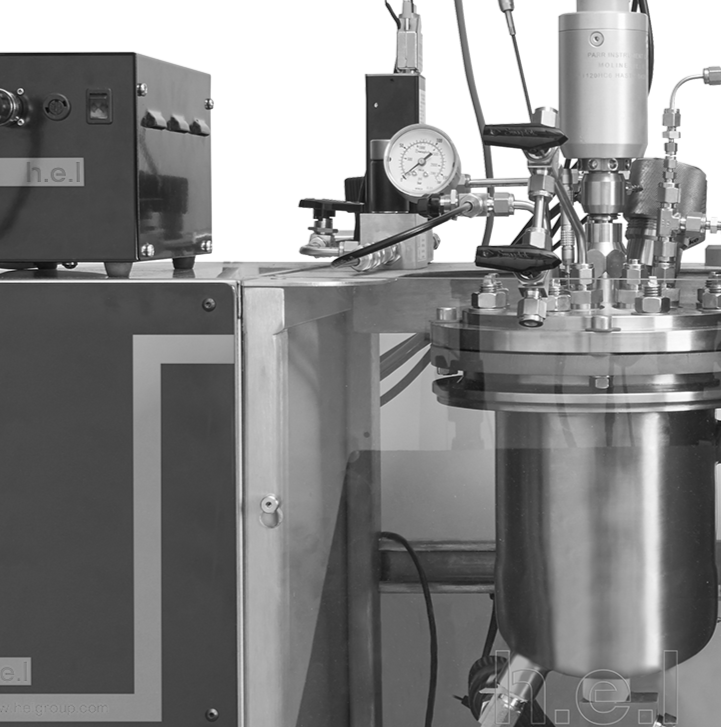
Calorimetric analysis of CO2 absorption in an aqueous N-methyldiethanolamine solution using H.E.L.’s Simular
The study of the CO2 absorption process in alkanolamines is crucial for enhancing gas sweetening and carbon capture technologies, which are essential in reducing greenhouse emissions. Alkanolamines, such as N-methyl diethanolamine (MDEA), are favored because of their thermodynamic and kinetic properties that improve CO2 capture efficiency. Understanding the calorimetry of these processes aids in optimizing absorption capacity and energy efficiency. Here, we show the thermodynamic behavior of CO2 absorption in a 30% MDEA solution using H.E.L’s Simular isothermal reaction calorimeter operated in power compensation mode. This finding highlights the potential of Simular as a powerful tool for optimizing CO2 absorption processes, contributing to effective and safe carbon capture technologies, and supporting global climate change mitigation efforts.
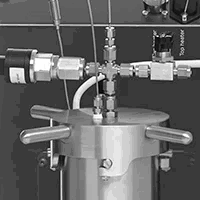
Achieving safe conditions for hydrogenation reaction using concentrated raw materials
Hydrogenation involves the addition of hydrogen gases into molecules, transforming bonds (e.g. C=C to C-C). Hydrogen is highly flammable and explosive, and as such, hydrogenation reactions and their products need to be tested in advance. In this application note, we present the thermal behavior of an alkane and alkene involved in the hydrogenation reaction measured in a Phi-TEC I. Results showed that the alkene was susceptible to decomposition reactions when it reached a temperature 238.8 oC, where the alkane did not display such behavior. Phi-TEC I is an adiabatic calorimeter that has shown its capabilities to detect thermal events in reactants involved in risky hydrogenation reactions.

Assessing the suitability of H.E.L’s BTC-500 for small battery testing
The constant pressure from the market to create more powerful batteries can result in unsafe situations. As a consequence, battery testing is crucial to obtain better batteries that can be operated safely. Adiabatic calorimetry is an incredibly powerful tool for identifying the temperature values at which a battery can be operated. The characterization of events, such as thermal runaways, can help identify the risks and establish mitigation strategies. However, choosing the right calorimeters can prove challenging because they are not sensitive enough for small batteries or cannot withstand battery failures. In this application note, we tested a very small battery (LP103035) in a large calorimeter (BTC-500).
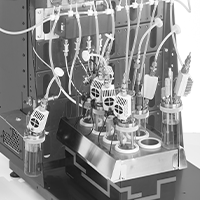
The use of a H.E.L BioXplorer in fermentations of Cupriavidus necator H16 at elevated pressure using CO2 as the sole carbon source
In this case study, the H.E.L BioXplorer was used to evaluate microbial fermentation in either autotrophic mode or heterotrophic mode.
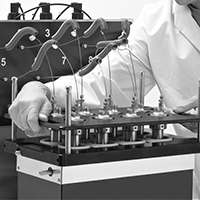
Investigating Hydrogenations at Different Catalyst Loadings using the ChemSCAN
In this study, a ChemSCAN is used to investigate the hydrogenation of nitrobenzene with a range of Pd/C catalyst loadings ranging from 4.0 mg to 9.3 mg. The experimental results showed that the hydrogenation reaction rate increases as the amount of catalyst in the reactor increases.


